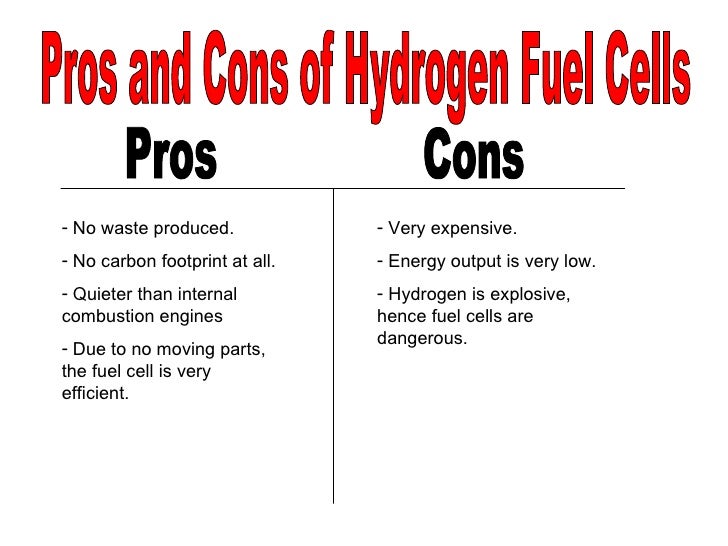
Fuel Cells In Chemistry Gcse . Learn about and revise chemical cells and fuel cells with this bbc bitesize gcse chemistry (edexcel) study guide. Revision notes on 5.4.2 fuel cells for the edexcel gcse chemistry syllabus, written by the chemistry experts at save my exams. A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode. Fuel cells produce electrical energy using a reaction between an external fuel source (often hydrogen) and oxygen. The fuel is added to the cell and then there is a constant supply of oxygen. The source of fuel could be an element such as. A fuel cell is an electrochemical cell that converts the chemical energy of a fuel, such as hydrogen, into electrical energy by reacting it with either pure oxygen or air. A chemical cell produces a voltage until one of the reactants is used up. Fuel cells have two main supplies. In order to make electricity, fuel cells will use an external source of fuel and oxygen.
from www.slideshare.net
A fuel cell is an electrochemical cell that converts the chemical energy of a fuel, such as hydrogen, into electrical energy by reacting it with either pure oxygen or air. A chemical cell produces a voltage until one of the reactants is used up. The source of fuel could be an element such as. Fuel cells produce electrical energy using a reaction between an external fuel source (often hydrogen) and oxygen. The fuel is added to the cell and then there is a constant supply of oxygen. A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode. Revision notes on 5.4.2 fuel cells for the edexcel gcse chemistry syllabus, written by the chemistry experts at save my exams. Fuel cells have two main supplies. In order to make electricity, fuel cells will use an external source of fuel and oxygen. Learn about and revise chemical cells and fuel cells with this bbc bitesize gcse chemistry (edexcel) study guide.
Fuel Cells
Fuel Cells In Chemistry Gcse The source of fuel could be an element such as. In order to make electricity, fuel cells will use an external source of fuel and oxygen. A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode. Revision notes on 5.4.2 fuel cells for the edexcel gcse chemistry syllabus, written by the chemistry experts at save my exams. Fuel cells have two main supplies. Learn about and revise chemical cells and fuel cells with this bbc bitesize gcse chemistry (edexcel) study guide. The source of fuel could be an element such as. Fuel cells produce electrical energy using a reaction between an external fuel source (often hydrogen) and oxygen. The fuel is added to the cell and then there is a constant supply of oxygen. A chemical cell produces a voltage until one of the reactants is used up. A fuel cell is an electrochemical cell that converts the chemical energy of a fuel, such as hydrogen, into electrical energy by reacting it with either pure oxygen or air.
From www.thesciencehive.co.uk
Fuel Cells (AQA) — the science sauce Fuel Cells In Chemistry Gcse The fuel is added to the cell and then there is a constant supply of oxygen. A fuel cell is an electrochemical cell that converts the chemical energy of a fuel, such as hydrogen, into electrical energy by reacting it with either pure oxygen or air. Learn about and revise chemical cells and fuel cells with this bbc bitesize gcse. Fuel Cells In Chemistry Gcse.
From www.youtube.com
Fuel Cells for AQA GCSE Chemistry YouTube Fuel Cells In Chemistry Gcse In order to make electricity, fuel cells will use an external source of fuel and oxygen. A fuel cell is an electrochemical cell that converts the chemical energy of a fuel, such as hydrogen, into electrical energy by reacting it with either pure oxygen or air. The fuel is added to the cell and then there is a constant supply. Fuel Cells In Chemistry Gcse.
From studymind.co.uk
Fuel Cells (GCSE Chemistry) Study Mind Fuel Cells In Chemistry Gcse Fuel cells have two main supplies. In order to make electricity, fuel cells will use an external source of fuel and oxygen. A fuel cell is an electrochemical cell that converts the chemical energy of a fuel, such as hydrogen, into electrical energy by reacting it with either pure oxygen or air. The fuel is added to the cell and. Fuel Cells In Chemistry Gcse.
From www.pinterest.co.uk
FREE GCSE Chemistry (Science) Hydrogen Fuel Cells Practice Exam Fuel Cells In Chemistry Gcse Learn about and revise chemical cells and fuel cells with this bbc bitesize gcse chemistry (edexcel) study guide. A chemical cell produces a voltage until one of the reactants is used up. In order to make electricity, fuel cells will use an external source of fuel and oxygen. The source of fuel could be an element such as. A fuel. Fuel Cells In Chemistry Gcse.
From www.youtube.com
Hydrogen & Fuel Cells Reactions Chemistry FuseSchool YouTube Fuel Cells In Chemistry Gcse Fuel cells produce electrical energy using a reaction between an external fuel source (often hydrogen) and oxygen. A fuel cell is an electrochemical cell that converts the chemical energy of a fuel, such as hydrogen, into electrical energy by reacting it with either pure oxygen or air. Learn about and revise chemical cells and fuel cells with this bbc bitesize. Fuel Cells In Chemistry Gcse.
From revisechemistry.uk
Organic chemistry (chemistry only) OCR Gateway C6 revisechemistry.uk Fuel Cells In Chemistry Gcse Fuel cells produce electrical energy using a reaction between an external fuel source (often hydrogen) and oxygen. A fuel cell is an electrochemical cell that converts the chemical energy of a fuel, such as hydrogen, into electrical energy by reacting it with either pure oxygen or air. A chemical cell produces a voltage until one of the reactants is used. Fuel Cells In Chemistry Gcse.
From pressbooks.openedmb.ca
Batteries and Fuel Cells Chemistry and the Environment Fuel Cells In Chemistry Gcse Fuel cells have two main supplies. In order to make electricity, fuel cells will use an external source of fuel and oxygen. The source of fuel could be an element such as. The fuel is added to the cell and then there is a constant supply of oxygen. A fuel cell is an electrochemical cell that converts the chemical energy. Fuel Cells In Chemistry Gcse.
From www.youtube.com
AQA GCSE Chemistry P1 Hydrogen Fuel Cells YouTube Fuel Cells In Chemistry Gcse A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode. The fuel is added to the cell and then there is a constant supply of oxygen. A fuel cell is an electrochemical cell that converts the chemical energy of a fuel, such as hydrogen, into electrical. Fuel Cells In Chemistry Gcse.
From mmerevise.co.uk
Electrochemical Cells Worksheets and Revision MME Fuel Cells In Chemistry Gcse Revision notes on 5.4.2 fuel cells for the edexcel gcse chemistry syllabus, written by the chemistry experts at save my exams. A chemical cell produces a voltage until one of the reactants is used up. Fuel cells produce electrical energy using a reaction between an external fuel source (often hydrogen) and oxygen. In order to make electricity, fuel cells will. Fuel Cells In Chemistry Gcse.
From chemistry.stackexchange.com
electrochemistry Electrode polarity in fuel cells Chemistry Stack Fuel Cells In Chemistry Gcse The source of fuel could be an element such as. A chemical cell produces a voltage until one of the reactants is used up. A fuel cell is an electrochemical cell that converts the chemical energy of a fuel, such as hydrogen, into electrical energy by reacting it with either pure oxygen or air. Learn about and revise chemical cells. Fuel Cells In Chemistry Gcse.
From www.youtube.com
The HydrogenOxygen Fuel Cell 91 GCSE Chemistry OCR, AQA, Edexcel Fuel Cells In Chemistry Gcse The source of fuel could be an element such as. In order to make electricity, fuel cells will use an external source of fuel and oxygen. A chemical cell produces a voltage until one of the reactants is used up. A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons. Fuel Cells In Chemistry Gcse.
From fuelcellscars.com
ALL ABOUT FUEL CELLS HOW DO THEY WORK Fuel Cells In Chemistry Gcse Revision notes on 5.4.2 fuel cells for the edexcel gcse chemistry syllabus, written by the chemistry experts at save my exams. Fuel cells produce electrical energy using a reaction between an external fuel source (often hydrogen) and oxygen. A fuel cell is an electrochemical cell that converts the chemical energy of a fuel, such as hydrogen, into electrical energy by. Fuel Cells In Chemistry Gcse.
From www.tes.com
Electrolysis and Fuel Cells GCSE Chemistry Teaching Resources Fuel Cells In Chemistry Gcse The fuel is added to the cell and then there is a constant supply of oxygen. A fuel cell is an electrochemical cell that converts the chemical energy of a fuel, such as hydrogen, into electrical energy by reacting it with either pure oxygen or air. A fuel cell is an electrochemical cell in which a fuel donates electrons at. Fuel Cells In Chemistry Gcse.
From www.youtube.com
An introduction to Fuel Cells (GCSE Chemistry) YouTube Fuel Cells In Chemistry Gcse A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode. The source of fuel could be an element such as. A chemical cell produces a voltage until one of the reactants is used up. In order to make electricity, fuel cells will use an external source. Fuel Cells In Chemistry Gcse.
From www.youtube.com
GCSE CHEMISTRY ELECTRO CHEMISTRY LESSON 11 simple voltaic cell Fuel Cells In Chemistry Gcse A chemical cell produces a voltage until one of the reactants is used up. A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode. In order to make electricity, fuel cells will use an external source of fuel and oxygen. A fuel cell is an electrochemical. Fuel Cells In Chemistry Gcse.
From techtimes12.com
Hydrogen gas cells vs. lithiumion batteries Powering EVs Techtimes12 Fuel Cells In Chemistry Gcse Learn about and revise chemical cells and fuel cells with this bbc bitesize gcse chemistry (edexcel) study guide. A fuel cell is an electrochemical cell that converts the chemical energy of a fuel, such as hydrogen, into electrical energy by reacting it with either pure oxygen or air. The fuel is added to the cell and then there is a. Fuel Cells In Chemistry Gcse.
From studiousguy.com
Fuel Cell Working Principle StudiousGuy Fuel Cells In Chemistry Gcse Learn about and revise chemical cells and fuel cells with this bbc bitesize gcse chemistry (edexcel) study guide. In order to make electricity, fuel cells will use an external source of fuel and oxygen. A fuel cell is an electrochemical cell that converts the chemical energy of a fuel, such as hydrogen, into electrical energy by reacting it with either. Fuel Cells In Chemistry Gcse.
From www.chfca.ca
About Fuel Cells CHFCA Fuel Cells In Chemistry Gcse A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode. Fuel cells have two main supplies. The source of fuel could be an element such as. Fuel cells produce electrical energy using a reaction between an external fuel source (often hydrogen) and oxygen. A fuel cell. Fuel Cells In Chemistry Gcse.
From www.tes.com
Fuel Cells GCSE AQA Teaching Resources Fuel Cells In Chemistry Gcse Learn about and revise chemical cells and fuel cells with this bbc bitesize gcse chemistry (edexcel) study guide. Revision notes on 5.4.2 fuel cells for the edexcel gcse chemistry syllabus, written by the chemistry experts at save my exams. A fuel cell is an electrochemical cell that converts the chemical energy of a fuel, such as hydrogen, into electrical energy. Fuel Cells In Chemistry Gcse.
From www.dataphysics-instruments.com
How measuring contact angles at high temperatures can help develop fuel Fuel Cells In Chemistry Gcse A fuel cell is an electrochemical cell that converts the chemical energy of a fuel, such as hydrogen, into electrical energy by reacting it with either pure oxygen or air. Revision notes on 5.4.2 fuel cells for the edexcel gcse chemistry syllabus, written by the chemistry experts at save my exams. Fuel cells produce electrical energy using a reaction between. Fuel Cells In Chemistry Gcse.
From stock.adobe.com
Fuel Cell Solid Oxide Diagram Stock Vector Adobe Stock Fuel Cells In Chemistry Gcse Fuel cells produce electrical energy using a reaction between an external fuel source (often hydrogen) and oxygen. Learn about and revise chemical cells and fuel cells with this bbc bitesize gcse chemistry (edexcel) study guide. A fuel cell is an electrochemical cell that converts the chemical energy of a fuel, such as hydrogen, into electrical energy by reacting it with. Fuel Cells In Chemistry Gcse.
From www.researchgate.net
6. Hydrogenoxygen fuel cell scheme. Download Scientific Diagram Fuel Cells In Chemistry Gcse The source of fuel could be an element such as. A chemical cell produces a voltage until one of the reactants is used up. Revision notes on 5.4.2 fuel cells for the edexcel gcse chemistry syllabus, written by the chemistry experts at save my exams. The fuel is added to the cell and then there is a constant supply of. Fuel Cells In Chemistry Gcse.
From www.tes.com
Chemical Cells and Fuel Cells GCSE AQA Chemistry HIGHER Teaching Fuel Cells In Chemistry Gcse Learn about and revise chemical cells and fuel cells with this bbc bitesize gcse chemistry (edexcel) study guide. Fuel cells produce electrical energy using a reaction between an external fuel source (often hydrogen) and oxygen. The source of fuel could be an element such as. A chemical cell produces a voltage until one of the reactants is used up. The. Fuel Cells In Chemistry Gcse.
From gradegorilla.com
GCSE Chemistry Cells Grade Gorilla Fuel Cells In Chemistry Gcse In order to make electricity, fuel cells will use an external source of fuel and oxygen. A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode. Revision notes on 5.4.2 fuel cells for the edexcel gcse chemistry syllabus, written by the chemistry experts at save my. Fuel Cells In Chemistry Gcse.
From www.youtube.com
Fuel Cell O level / IGCSE Chemistry YouTube Fuel Cells In Chemistry Gcse The fuel is added to the cell and then there is a constant supply of oxygen. Fuel cells produce electrical energy using a reaction between an external fuel source (often hydrogen) and oxygen. A fuel cell is an electrochemical cell that converts the chemical energy of a fuel, such as hydrogen, into electrical energy by reacting it with either pure. Fuel Cells In Chemistry Gcse.
From www.youtube.com
GCSE Chemistry Fuel Cells YouTube Fuel Cells In Chemistry Gcse Revision notes on 5.4.2 fuel cells for the edexcel gcse chemistry syllabus, written by the chemistry experts at save my exams. In order to make electricity, fuel cells will use an external source of fuel and oxygen. Learn about and revise chemical cells and fuel cells with this bbc bitesize gcse chemistry (edexcel) study guide. The fuel is added to. Fuel Cells In Chemistry Gcse.
From www.tes.com
Chemical Cells and Fuel Cells Mind map for AQA GCSE Chemistry for 2018 Fuel Cells In Chemistry Gcse Revision notes on 5.4.2 fuel cells for the edexcel gcse chemistry syllabus, written by the chemistry experts at save my exams. Fuel cells have two main supplies. A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode. A fuel cell is an electrochemical cell that converts. Fuel Cells In Chemistry Gcse.
From www.tes.com
KS4 GCSE Chemistry AQA C7 6 Fuel cells PPT only Teaching Resources Fuel Cells In Chemistry Gcse A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode. The source of fuel could be an element such as. A fuel cell is an electrochemical cell that converts the chemical energy of a fuel, such as hydrogen, into electrical energy by reacting it with either. Fuel Cells In Chemistry Gcse.
From www.thinkswap.com
Fuel Cells Chemistry Year 12 VCE Thinkswap Fuel Cells In Chemistry Gcse A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode. A chemical cell produces a voltage until one of the reactants is used up. Learn about and revise chemical cells and fuel cells with this bbc bitesize gcse chemistry (edexcel) study guide. The fuel is added. Fuel Cells In Chemistry Gcse.
From www.slideshare.net
Fuel Cells Fuel Cells In Chemistry Gcse A fuel cell is an electrochemical cell that converts the chemical energy of a fuel, such as hydrogen, into electrical energy by reacting it with either pure oxygen or air. In order to make electricity, fuel cells will use an external source of fuel and oxygen. Revision notes on 5.4.2 fuel cells for the edexcel gcse chemistry syllabus, written by. Fuel Cells In Chemistry Gcse.
From leehamnews.com
Bjorn’s Corner The challenges of hydrogen. Part 22. Hydrogen fuel Fuel Cells In Chemistry Gcse Fuel cells have two main supplies. The fuel is added to the cell and then there is a constant supply of oxygen. Revision notes on 5.4.2 fuel cells for the edexcel gcse chemistry syllabus, written by the chemistry experts at save my exams. A fuel cell is an electrochemical cell that converts the chemical energy of a fuel, such as. Fuel Cells In Chemistry Gcse.
From www.vhv.rs
Fuel Cell Block Diagram Hydrogen Fuel Cells Aqa Chemistry Gcse, HD Fuel Cells In Chemistry Gcse Revision notes on 5.4.2 fuel cells for the edexcel gcse chemistry syllabus, written by the chemistry experts at save my exams. A fuel cell is an electrochemical cell that converts the chemical energy of a fuel, such as hydrogen, into electrical energy by reacting it with either pure oxygen or air. Learn about and revise chemical cells and fuel cells. Fuel Cells In Chemistry Gcse.
From www.vrogue.co
Free Gcse Chemistry Science Hydrogen Fuel Cells Pract vrogue.co Fuel Cells In Chemistry Gcse In order to make electricity, fuel cells will use an external source of fuel and oxygen. The source of fuel could be an element such as. A chemical cell produces a voltage until one of the reactants is used up. Revision notes on 5.4.2 fuel cells for the edexcel gcse chemistry syllabus, written by the chemistry experts at save my. Fuel Cells In Chemistry Gcse.
From www.youtube.com
GCSE Chemistry Revision "Fuel Cells" (Triple) YouTube Fuel Cells In Chemistry Gcse A fuel cell is an electrochemical cell that converts the chemical energy of a fuel, such as hydrogen, into electrical energy by reacting it with either pure oxygen or air. Fuel cells have two main supplies. In order to make electricity, fuel cells will use an external source of fuel and oxygen. The fuel is added to the cell and. Fuel Cells In Chemistry Gcse.
From www.savemyexams.com
Fuel Cells SL IB Chemistry Revision Notes 2025 Fuel Cells In Chemistry Gcse The source of fuel could be an element such as. A chemical cell produces a voltage until one of the reactants is used up. A fuel cell is an electrochemical cell that converts the chemical energy of a fuel, such as hydrogen, into electrical energy by reacting it with either pure oxygen or air. Revision notes on 5.4.2 fuel cells. Fuel Cells In Chemistry Gcse.