Is The Equilibrium Constant Always The Same . Express reaction quotients for chemical equations representing homogeneous and. the equilibrium constant always has the same value (provided you don't change the temperature), irrespective of the amounts. this page looks at the relationship between equilibrium constants and le chatelier's principle. by its definition, the magnitude of an equilibrium constant explicitly reflects the composition of a reaction mixture at equilibrium, and it may be. the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium. when a mixture of reactants and products of a reaction reaches equilibrium at a given temperature, its reaction quotient always. Students often get confused about how it is possible. that is, at a given temperature, the equilibrium constant for a reaction always has the same value, even though the specific concentrations of.
from general.chemistrysteps.com
Express reaction quotients for chemical equations representing homogeneous and. Students often get confused about how it is possible. by its definition, the magnitude of an equilibrium constant explicitly reflects the composition of a reaction mixture at equilibrium, and it may be. this page looks at the relationship between equilibrium constants and le chatelier's principle. when a mixture of reactants and products of a reaction reaches equilibrium at a given temperature, its reaction quotient always. the equilibrium constant always has the same value (provided you don't change the temperature), irrespective of the amounts. the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium. that is, at a given temperature, the equilibrium constant for a reaction always has the same value, even though the specific concentrations of.
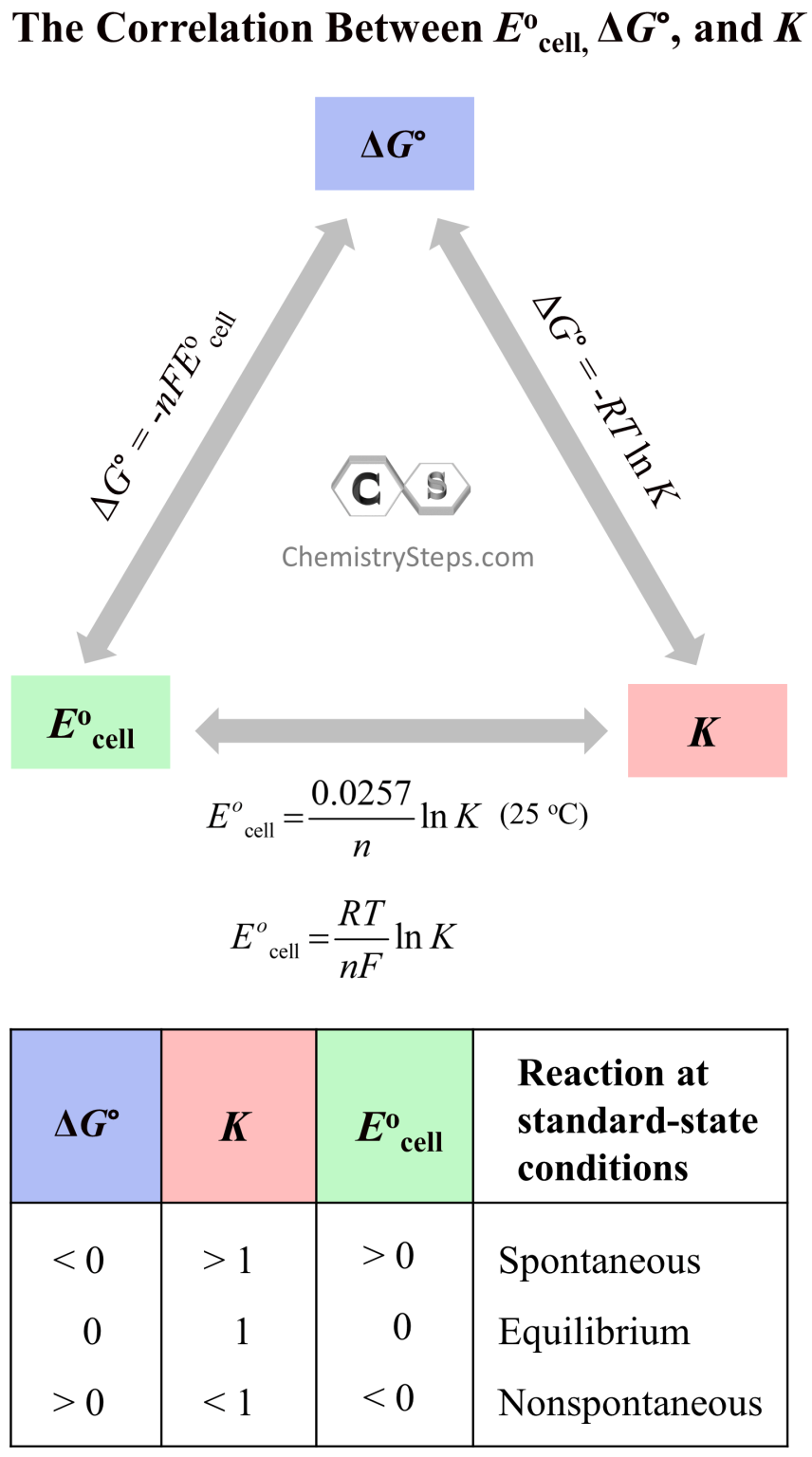
Cell Potential, Free Energy, and Equilibrium Constant Chemistry Steps
Is The Equilibrium Constant Always The Same when a mixture of reactants and products of a reaction reaches equilibrium at a given temperature, its reaction quotient always. Express reaction quotients for chemical equations representing homogeneous and. the equilibrium constant always has the same value (provided you don't change the temperature), irrespective of the amounts. by its definition, the magnitude of an equilibrium constant explicitly reflects the composition of a reaction mixture at equilibrium, and it may be. that is, at a given temperature, the equilibrium constant for a reaction always has the same value, even though the specific concentrations of. when a mixture of reactants and products of a reaction reaches equilibrium at a given temperature, its reaction quotient always. the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium. Students often get confused about how it is possible. this page looks at the relationship between equilibrium constants and le chatelier's principle.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID Is The Equilibrium Constant Always The Same when a mixture of reactants and products of a reaction reaches equilibrium at a given temperature, its reaction quotient always. the equilibrium constant always has the same value (provided you don't change the temperature), irrespective of the amounts. that is, at a given temperature, the equilibrium constant for a reaction always has the same value, even though. Is The Equilibrium Constant Always The Same.
From solvedlib.com
Calculate equilibrium constant for the reaction using… SolvedLib Is The Equilibrium Constant Always The Same by its definition, the magnitude of an equilibrium constant explicitly reflects the composition of a reaction mixture at equilibrium, and it may be. Students often get confused about how it is possible. that is, at a given temperature, the equilibrium constant for a reaction always has the same value, even though the specific concentrations of. the equilibrium. Is The Equilibrium Constant Always The Same.
From general.chemistrysteps.com
Cell Potential, Free Energy, and Equilibrium Constant Chemistry Steps Is The Equilibrium Constant Always The Same that is, at a given temperature, the equilibrium constant for a reaction always has the same value, even though the specific concentrations of. Express reaction quotients for chemical equations representing homogeneous and. Students often get confused about how it is possible. the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium. . Is The Equilibrium Constant Always The Same.
From www.slideserve.com
PPT Chapter 13 Chemical Equilibrium PowerPoint Presentation, free Is The Equilibrium Constant Always The Same Express reaction quotients for chemical equations representing homogeneous and. when a mixture of reactants and products of a reaction reaches equilibrium at a given temperature, its reaction quotient always. the equilibrium constant always has the same value (provided you don't change the temperature), irrespective of the amounts. the equilibrium constant, k, expresses the relationship between products and. Is The Equilibrium Constant Always The Same.
From www.youtube.com
Calculate the equilibrium constant YouTube Is The Equilibrium Constant Always The Same Express reaction quotients for chemical equations representing homogeneous and. the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium. Students often get confused about how it is possible. by its definition, the magnitude of an equilibrium constant explicitly reflects the composition of a reaction mixture at equilibrium, and it may be. . Is The Equilibrium Constant Always The Same.
From studylib.net
Equilibrium for a general reaction Is The Equilibrium Constant Always The Same the equilibrium constant always has the same value (provided you don't change the temperature), irrespective of the amounts. by its definition, the magnitude of an equilibrium constant explicitly reflects the composition of a reaction mixture at equilibrium, and it may be. that is, at a given temperature, the equilibrium constant for a reaction always has the same. Is The Equilibrium Constant Always The Same.
From www.youtube.com
Week 7 1. What are the units of the equilibrium constant, K? YouTube Is The Equilibrium Constant Always The Same Students often get confused about how it is possible. the equilibrium constant always has the same value (provided you don't change the temperature), irrespective of the amounts. by its definition, the magnitude of an equilibrium constant explicitly reflects the composition of a reaction mixture at equilibrium, and it may be. this page looks at the relationship between. Is The Equilibrium Constant Always The Same.
From www.slideserve.com
PPT The Equilibrium Constant Expression PowerPoint Presentation, free Is The Equilibrium Constant Always The Same Express reaction quotients for chemical equations representing homogeneous and. that is, at a given temperature, the equilibrium constant for a reaction always has the same value, even though the specific concentrations of. by its definition, the magnitude of an equilibrium constant explicitly reflects the composition of a reaction mixture at equilibrium, and it may be. when a. Is The Equilibrium Constant Always The Same.
From www.youtube.com
Using equilibrium constants in terms of concentrations and partial Is The Equilibrium Constant Always The Same that is, at a given temperature, the equilibrium constant for a reaction always has the same value, even though the specific concentrations of. the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium. by its definition, the magnitude of an equilibrium constant explicitly reflects the composition of a reaction mixture at. Is The Equilibrium Constant Always The Same.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID Is The Equilibrium Constant Always The Same that is, at a given temperature, the equilibrium constant for a reaction always has the same value, even though the specific concentrations of. this page looks at the relationship between equilibrium constants and le chatelier's principle. the equilibrium constant always has the same value (provided you don't change the temperature), irrespective of the amounts. Students often get. Is The Equilibrium Constant Always The Same.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID Is The Equilibrium Constant Always The Same by its definition, the magnitude of an equilibrium constant explicitly reflects the composition of a reaction mixture at equilibrium, and it may be. Students often get confused about how it is possible. the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium. this page looks at the relationship between equilibrium constants. Is The Equilibrium Constant Always The Same.
From www.pdfprof.com
chemical equilibrium finding a constant Is The Equilibrium Constant Always The Same the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium. Students often get confused about how it is possible. by its definition, the magnitude of an equilibrium constant explicitly reflects the composition of a reaction mixture at equilibrium, and it may be. Express reaction quotients for chemical equations representing homogeneous and. . Is The Equilibrium Constant Always The Same.
From www.youtube.com
Understanding The Equilibrium Constant, Kp (A2 Chemistry) YouTube Is The Equilibrium Constant Always The Same this page looks at the relationship between equilibrium constants and le chatelier's principle. the equilibrium constant always has the same value (provided you don't change the temperature), irrespective of the amounts. by its definition, the magnitude of an equilibrium constant explicitly reflects the composition of a reaction mixture at equilibrium, and it may be. Express reaction quotients. Is The Equilibrium Constant Always The Same.
From haipernews.com
How To Calculate The Equilibrium Constant Haiper Is The Equilibrium Constant Always The Same the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium. Express reaction quotients for chemical equations representing homogeneous and. the equilibrium constant always has the same value (provided you don't change the temperature), irrespective of the amounts. when a mixture of reactants and products of a reaction reaches equilibrium at a. Is The Equilibrium Constant Always The Same.
From www.youtube.com
EQUILIBRIUM CONSTANT AND UNIT PART 1 / CONTEXT OF CHEMISTRY YouTube Is The Equilibrium Constant Always The Same the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium. Express reaction quotients for chemical equations representing homogeneous and. this page looks at the relationship between equilibrium constants and le chatelier's principle. Students often get confused about how it is possible. the equilibrium constant always has the same value (provided you. Is The Equilibrium Constant Always The Same.
From facts.net
13 Fascinating Facts About Equilibrium Constant (Kc) Is The Equilibrium Constant Always The Same when a mixture of reactants and products of a reaction reaches equilibrium at a given temperature, its reaction quotient always. Students often get confused about how it is possible. this page looks at the relationship between equilibrium constants and le chatelier's principle. the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at. Is The Equilibrium Constant Always The Same.
From slideplayer.com
Introduction to the Equilibrium Constant ppt download Is The Equilibrium Constant Always The Same Express reaction quotients for chemical equations representing homogeneous and. this page looks at the relationship between equilibrium constants and le chatelier's principle. when a mixture of reactants and products of a reaction reaches equilibrium at a given temperature, its reaction quotient always. Students often get confused about how it is possible. by its definition, the magnitude of. Is The Equilibrium Constant Always The Same.
From general.chemistrysteps.com
Equilibrium Constant Chemistry Steps Is The Equilibrium Constant Always The Same that is, at a given temperature, the equilibrium constant for a reaction always has the same value, even though the specific concentrations of. the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium. this page looks at the relationship between equilibrium constants and le chatelier's principle. when a mixture of. Is The Equilibrium Constant Always The Same.
From www.physicsforums.com
Solving Equilibrium Constant Is The Equilibrium Constant Always The Same when a mixture of reactants and products of a reaction reaches equilibrium at a given temperature, its reaction quotient always. that is, at a given temperature, the equilibrium constant for a reaction always has the same value, even though the specific concentrations of. this page looks at the relationship between equilibrium constants and le chatelier's principle. . Is The Equilibrium Constant Always The Same.
From learningchemistryeasily.blogspot.com
Learning Chemistry Easily Chemical Equilibrium Theory, An Introduction Is The Equilibrium Constant Always The Same by its definition, the magnitude of an equilibrium constant explicitly reflects the composition of a reaction mixture at equilibrium, and it may be. the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium. Express reaction quotients for chemical equations representing homogeneous and. Students often get confused about how it is possible. . Is The Equilibrium Constant Always The Same.
From www.slideserve.com
PPT Chapter 19 PowerPoint Presentation ID733334 Is The Equilibrium Constant Always The Same Students often get confused about how it is possible. the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium. this page looks at the relationship between equilibrium constants and le chatelier's principle. when a mixture of reactants and products of a reaction reaches equilibrium at a given temperature, its reaction quotient. Is The Equilibrium Constant Always The Same.
From www.slideserve.com
PPT Equilibrium PowerPoint Presentation, free download ID1533932 Is The Equilibrium Constant Always The Same when a mixture of reactants and products of a reaction reaches equilibrium at a given temperature, its reaction quotient always. Students often get confused about how it is possible. that is, at a given temperature, the equilibrium constant for a reaction always has the same value, even though the specific concentrations of. this page looks at the. Is The Equilibrium Constant Always The Same.
From www.slideserve.com
PPT Chapter 15 Chemical Equilibrium PowerPoint Presentation, free Is The Equilibrium Constant Always The Same that is, at a given temperature, the equilibrium constant for a reaction always has the same value, even though the specific concentrations of. Students often get confused about how it is possible. Express reaction quotients for chemical equations representing homogeneous and. the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium. . Is The Equilibrium Constant Always The Same.
From www.researchgate.net
Reactions and equilibrium constant equations. Download Scientific Diagram Is The Equilibrium Constant Always The Same by its definition, the magnitude of an equilibrium constant explicitly reflects the composition of a reaction mixture at equilibrium, and it may be. this page looks at the relationship between equilibrium constants and le chatelier's principle. when a mixture of reactants and products of a reaction reaches equilibrium at a given temperature, its reaction quotient always. Students. Is The Equilibrium Constant Always The Same.
From thechemistrynotes.com
Equilibrium Constant Definition, Characteristics, and Calculations Is The Equilibrium Constant Always The Same that is, at a given temperature, the equilibrium constant for a reaction always has the same value, even though the specific concentrations of. this page looks at the relationship between equilibrium constants and le chatelier's principle. when a mixture of reactants and products of a reaction reaches equilibrium at a given temperature, its reaction quotient always. . Is The Equilibrium Constant Always The Same.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID Is The Equilibrium Constant Always The Same the equilibrium constant always has the same value (provided you don't change the temperature), irrespective of the amounts. Express reaction quotients for chemical equations representing homogeneous and. the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium. that is, at a given temperature, the equilibrium constant for a reaction always has. Is The Equilibrium Constant Always The Same.
From www.youtube.com
The equilibrium constant Kp YouTube Is The Equilibrium Constant Always The Same Students often get confused about how it is possible. the equilibrium constant always has the same value (provided you don't change the temperature), irrespective of the amounts. Express reaction quotients for chemical equations representing homogeneous and. that is, at a given temperature, the equilibrium constant for a reaction always has the same value, even though the specific concentrations. Is The Equilibrium Constant Always The Same.
From www.youtube.com
Relationship Between the Equilibrium Constants YouTube Is The Equilibrium Constant Always The Same by its definition, the magnitude of an equilibrium constant explicitly reflects the composition of a reaction mixture at equilibrium, and it may be. that is, at a given temperature, the equilibrium constant for a reaction always has the same value, even though the specific concentrations of. when a mixture of reactants and products of a reaction reaches. Is The Equilibrium Constant Always The Same.
From www.slideserve.com
PPT Equilibrium Chemistry PowerPoint Presentation, free download ID Is The Equilibrium Constant Always The Same this page looks at the relationship between equilibrium constants and le chatelier's principle. that is, at a given temperature, the equilibrium constant for a reaction always has the same value, even though the specific concentrations of. when a mixture of reactants and products of a reaction reaches equilibrium at a given temperature, its reaction quotient always. . Is The Equilibrium Constant Always The Same.
From www.slideserve.com
PPT Ch. 13 Chemical Equilibrium PowerPoint Presentation, free Is The Equilibrium Constant Always The Same this page looks at the relationship between equilibrium constants and le chatelier's principle. the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium. the equilibrium constant always has the same value (provided you don't change the temperature), irrespective of the amounts. when a mixture of reactants and products of a. Is The Equilibrium Constant Always The Same.
From www.vrogue.co
The Equilibrium Constant Kp Of The Reaction 2so2 O2 2 vrogue.co Is The Equilibrium Constant Always The Same by its definition, the magnitude of an equilibrium constant explicitly reflects the composition of a reaction mixture at equilibrium, and it may be. Express reaction quotients for chemical equations representing homogeneous and. this page looks at the relationship between equilibrium constants and le chatelier's principle. the equilibrium constant, k, expresses the relationship between products and reactants of. Is The Equilibrium Constant Always The Same.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID Is The Equilibrium Constant Always The Same by its definition, the magnitude of an equilibrium constant explicitly reflects the composition of a reaction mixture at equilibrium, and it may be. this page looks at the relationship between equilibrium constants and le chatelier's principle. the equilibrium constant always has the same value (provided you don't change the temperature), irrespective of the amounts. when a. Is The Equilibrium Constant Always The Same.
From www.slideserve.com
PPT THE EQUILIBRIUM CONSTANT PowerPoint Presentation, free download Is The Equilibrium Constant Always The Same the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium. that is, at a given temperature, the equilibrium constant for a reaction always has the same value, even though the specific concentrations of. this page looks at the relationship between equilibrium constants and le chatelier's principle. Students often get confused about. Is The Equilibrium Constant Always The Same.
From www.masterorganicchemistry.com
Equilibrium and Energy Relationships Master Organic Chemistry Is The Equilibrium Constant Always The Same the equilibrium constant always has the same value (provided you don't change the temperature), irrespective of the amounts. this page looks at the relationship between equilibrium constants and le chatelier's principle. that is, at a given temperature, the equilibrium constant for a reaction always has the same value, even though the specific concentrations of. the equilibrium. Is The Equilibrium Constant Always The Same.
From www.slideserve.com
PPT The Equilibrium Constant PowerPoint Presentation, free download Is The Equilibrium Constant Always The Same Express reaction quotients for chemical equations representing homogeneous and. the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium. by its definition, the magnitude of an equilibrium constant explicitly reflects the composition of a reaction mixture at equilibrium, and it may be. the equilibrium constant always has the same value (provided. Is The Equilibrium Constant Always The Same.