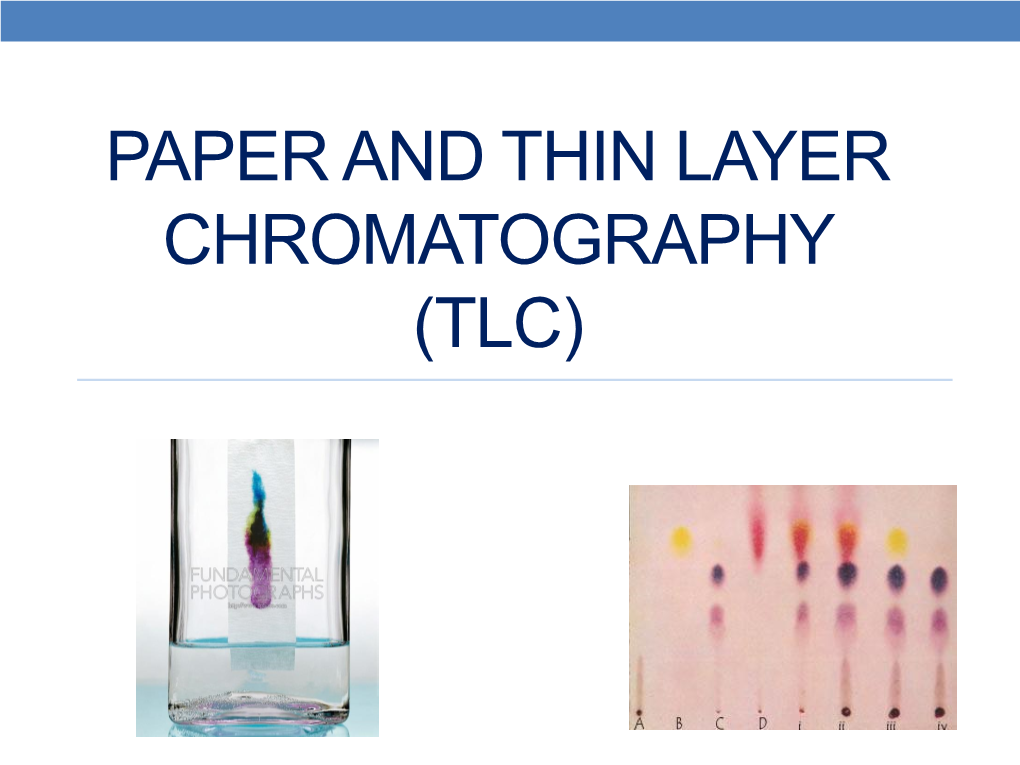
Chromatography Paper Objectives . in paper chromatography, a specialized paper acts as the stationary phase, while a liquid solvent is the mobile phase. As the solvent moves up through capillary action, it carries To use paper chromatography to identify whether certain food dyes are pure substances or. this chapter discusses planar chromatography and column chromatography. • separate mixtures of compounds, using the technique of paper chromatography. The mixture to be analyzed is applied to the paper. paper chromatography objectives c2.7 explain the experimental techniques for separation of mixtures by: A technique of separation and identification section 1: known and unknown solutions of the metal ions \(\ce{ag^{+}}\), \(\ce{fe^{3+}}\), \(\ce{co^{2+}}\), \(\ce{cu^{2+}}\) and.
from docslib.org
paper chromatography objectives c2.7 explain the experimental techniques for separation of mixtures by: The mixture to be analyzed is applied to the paper. To use paper chromatography to identify whether certain food dyes are pure substances or. this chapter discusses planar chromatography and column chromatography. A technique of separation and identification section 1: known and unknown solutions of the metal ions \(\ce{ag^{+}}\), \(\ce{fe^{3+}}\), \(\ce{co^{2+}}\), \(\ce{cu^{2+}}\) and. in paper chromatography, a specialized paper acts as the stationary phase, while a liquid solvent is the mobile phase. • separate mixtures of compounds, using the technique of paper chromatography. As the solvent moves up through capillary action, it carries
PAPER and THIN LAYER CHROMATOGRAPHY (TLC) Objectives DocsLib
Chromatography Paper Objectives As the solvent moves up through capillary action, it carries To use paper chromatography to identify whether certain food dyes are pure substances or. in paper chromatography, a specialized paper acts as the stationary phase, while a liquid solvent is the mobile phase. known and unknown solutions of the metal ions \(\ce{ag^{+}}\), \(\ce{fe^{3+}}\), \(\ce{co^{2+}}\), \(\ce{cu^{2+}}\) and. paper chromatography objectives c2.7 explain the experimental techniques for separation of mixtures by: A technique of separation and identification section 1: The mixture to be analyzed is applied to the paper. this chapter discusses planar chromatography and column chromatography. • separate mixtures of compounds, using the technique of paper chromatography. As the solvent moves up through capillary action, it carries
From studylib.net
Paper Chromatography Chromatography Paper Objectives known and unknown solutions of the metal ions \(\ce{ag^{+}}\), \(\ce{fe^{3+}}\), \(\ce{co^{2+}}\), \(\ce{cu^{2+}}\) and. As the solvent moves up through capillary action, it carries The mixture to be analyzed is applied to the paper. • separate mixtures of compounds, using the technique of paper chromatography. in paper chromatography, a specialized paper acts as the stationary phase, while a liquid. Chromatography Paper Objectives.
From owlcation.com
What Is Paper Chromatography Principle, Types, and Uses Owlcation Chromatography Paper Objectives this chapter discusses planar chromatography and column chromatography. paper chromatography objectives c2.7 explain the experimental techniques for separation of mixtures by: known and unknown solutions of the metal ions \(\ce{ag^{+}}\), \(\ce{fe^{3+}}\), \(\ce{co^{2+}}\), \(\ce{cu^{2+}}\) and. As the solvent moves up through capillary action, it carries To use paper chromatography to identify whether certain food dyes are pure substances. Chromatography Paper Objectives.
From studylib.net
Paper Chromatography Chromatography Paper Objectives • separate mixtures of compounds, using the technique of paper chromatography. The mixture to be analyzed is applied to the paper. A technique of separation and identification section 1: this chapter discusses planar chromatography and column chromatography. in paper chromatography, a specialized paper acts as the stationary phase, while a liquid solvent is the mobile phase. As the. Chromatography Paper Objectives.
From thechemistrynotes.com
Paper Chromatography Chromatography Paper Objectives A technique of separation and identification section 1: known and unknown solutions of the metal ions \(\ce{ag^{+}}\), \(\ce{fe^{3+}}\), \(\ce{co^{2+}}\), \(\ce{cu^{2+}}\) and. • separate mixtures of compounds, using the technique of paper chromatography. The mixture to be analyzed is applied to the paper. As the solvent moves up through capillary action, it carries paper chromatography objectives c2.7 explain the. Chromatography Paper Objectives.
From mungfali.com
Paper Chromatography Labelled Diagram Chromatography Paper Objectives As the solvent moves up through capillary action, it carries The mixture to be analyzed is applied to the paper. • separate mixtures of compounds, using the technique of paper chromatography. this chapter discusses planar chromatography and column chromatography. paper chromatography objectives c2.7 explain the experimental techniques for separation of mixtures by: To use paper chromatography to identify. Chromatography Paper Objectives.
From microbenotes.com
Paper Chromatography Definition, Types, Principle, Steps, Uses Chromatography Paper Objectives The mixture to be analyzed is applied to the paper. in paper chromatography, a specialized paper acts as the stationary phase, while a liquid solvent is the mobile phase. this chapter discusses planar chromatography and column chromatography. • separate mixtures of compounds, using the technique of paper chromatography. known and unknown solutions of the metal ions \(\ce{ag^{+}}\),. Chromatography Paper Objectives.
From www.studocu.com
Paper Chromatography it is lecture notes PAPER CHROMATOGRAPHY paper Chromatography Paper Objectives As the solvent moves up through capillary action, it carries The mixture to be analyzed is applied to the paper. this chapter discusses planar chromatography and column chromatography. paper chromatography objectives c2.7 explain the experimental techniques for separation of mixtures by: known and unknown solutions of the metal ions \(\ce{ag^{+}}\), \(\ce{fe^{3+}}\), \(\ce{co^{2+}}\), \(\ce{cu^{2+}}\) and. • separate mixtures. Chromatography Paper Objectives.
From www.youtube.com
Paper chromatography/Radial paper chromatography (Principle, procedure Chromatography Paper Objectives To use paper chromatography to identify whether certain food dyes are pure substances or. in paper chromatography, a specialized paper acts as the stationary phase, while a liquid solvent is the mobile phase. The mixture to be analyzed is applied to the paper. this chapter discusses planar chromatography and column chromatography. A technique of separation and identification section. Chromatography Paper Objectives.
From www.slideserve.com
PPT Paper Chromatography PowerPoint Presentation, free download ID Chromatography Paper Objectives paper chromatography objectives c2.7 explain the experimental techniques for separation of mixtures by: A technique of separation and identification section 1: this chapter discusses planar chromatography and column chromatography. As the solvent moves up through capillary action, it carries The mixture to be analyzed is applied to the paper. To use paper chromatography to identify whether certain food. Chromatography Paper Objectives.
From studylib.net
Paper Chromatography Purpose and Objectives Chromatography Paper Objectives • separate mixtures of compounds, using the technique of paper chromatography. paper chromatography objectives c2.7 explain the experimental techniques for separation of mixtures by: known and unknown solutions of the metal ions \(\ce{ag^{+}}\), \(\ce{fe^{3+}}\), \(\ce{co^{2+}}\), \(\ce{cu^{2+}}\) and. A technique of separation and identification section 1: in paper chromatography, a specialized paper acts as the stationary phase, while. Chromatography Paper Objectives.
From www.studypool.com
SOLUTION Igcse chemistry introduction to simple paper chromatography Chromatography Paper Objectives • separate mixtures of compounds, using the technique of paper chromatography. known and unknown solutions of the metal ions \(\ce{ag^{+}}\), \(\ce{fe^{3+}}\), \(\ce{co^{2+}}\), \(\ce{cu^{2+}}\) and. in paper chromatography, a specialized paper acts as the stationary phase, while a liquid solvent is the mobile phase. The mixture to be analyzed is applied to the paper. this chapter discusses planar. Chromatography Paper Objectives.
From dokumen.tips
(PPT) Paper Chromatography ppt DOKUMEN.TIPS Chromatography Paper Objectives To use paper chromatography to identify whether certain food dyes are pure substances or. known and unknown solutions of the metal ions \(\ce{ag^{+}}\), \(\ce{fe^{3+}}\), \(\ce{co^{2+}}\), \(\ce{cu^{2+}}\) and. this chapter discusses planar chromatography and column chromatography. paper chromatography objectives c2.7 explain the experimental techniques for separation of mixtures by: As the solvent moves up through capillary action, it. Chromatography Paper Objectives.
From www.youtube.com
Paper chromatography Principle Procedure Development techniques Chromatography Paper Objectives A technique of separation and identification section 1: known and unknown solutions of the metal ions \(\ce{ag^{+}}\), \(\ce{fe^{3+}}\), \(\ce{co^{2+}}\), \(\ce{cu^{2+}}\) and. paper chromatography objectives c2.7 explain the experimental techniques for separation of mixtures by: in paper chromatography, a specialized paper acts as the stationary phase, while a liquid solvent is the mobile phase. this chapter discusses. Chromatography Paper Objectives.
From owlcation.com
What Is Paper Chromatography and How Does It Work? Owlcation Chromatography Paper Objectives • separate mixtures of compounds, using the technique of paper chromatography. known and unknown solutions of the metal ions \(\ce{ag^{+}}\), \(\ce{fe^{3+}}\), \(\ce{co^{2+}}\), \(\ce{cu^{2+}}\) and. in paper chromatography, a specialized paper acts as the stationary phase, while a liquid solvent is the mobile phase. this chapter discusses planar chromatography and column chromatography. As the solvent moves up through. Chromatography Paper Objectives.
From testbook.com
Paper Chromatography Learn Principle, Diagram, Types & Uses Chromatography Paper Objectives • separate mixtures of compounds, using the technique of paper chromatography. paper chromatography objectives c2.7 explain the experimental techniques for separation of mixtures by: in paper chromatography, a specialized paper acts as the stationary phase, while a liquid solvent is the mobile phase. As the solvent moves up through capillary action, it carries A technique of separation and. Chromatography Paper Objectives.
From www.studocu.com
Paper Chromatography of Gel Ink Pens Paper Chromatography of Gel Ink Chromatography Paper Objectives To use paper chromatography to identify whether certain food dyes are pure substances or. A technique of separation and identification section 1: The mixture to be analyzed is applied to the paper. in paper chromatography, a specialized paper acts as the stationary phase, while a liquid solvent is the mobile phase. • separate mixtures of compounds, using the technique. Chromatography Paper Objectives.
From www.studypool.com
SOLUTION paper chromatography (notes) Studypool Chromatography Paper Objectives The mixture to be analyzed is applied to the paper. To use paper chromatography to identify whether certain food dyes are pure substances or. paper chromatography objectives c2.7 explain the experimental techniques for separation of mixtures by: known and unknown solutions of the metal ions \(\ce{ag^{+}}\), \(\ce{fe^{3+}}\), \(\ce{co^{2+}}\), \(\ce{cu^{2+}}\) and. this chapter discusses planar chromatography and column. Chromatography Paper Objectives.
From www.studocu.com
Lab 5 aaaaa LAB 5 PAPER CHROMATOGRAPHY Objectives To gain Chromatography Paper Objectives As the solvent moves up through capillary action, it carries A technique of separation and identification section 1: in paper chromatography, a specialized paper acts as the stationary phase, while a liquid solvent is the mobile phase. paper chromatography objectives c2.7 explain the experimental techniques for separation of mixtures by: known and unknown solutions of the metal. Chromatography Paper Objectives.
From docslib.org
PAPER and THIN LAYER CHROMATOGRAPHY (TLC) Objectives DocsLib Chromatography Paper Objectives • separate mixtures of compounds, using the technique of paper chromatography. As the solvent moves up through capillary action, it carries A technique of separation and identification section 1: paper chromatography objectives c2.7 explain the experimental techniques for separation of mixtures by: known and unknown solutions of the metal ions \(\ce{ag^{+}}\), \(\ce{fe^{3+}}\), \(\ce{co^{2+}}\), \(\ce{cu^{2+}}\) and. The mixture to. Chromatography Paper Objectives.
From haslag.us
Paper Chromatography Catherine Haslag Chromatography Paper Objectives As the solvent moves up through capillary action, it carries A technique of separation and identification section 1: • separate mixtures of compounds, using the technique of paper chromatography. this chapter discusses planar chromatography and column chromatography. known and unknown solutions of the metal ions \(\ce{ag^{+}}\), \(\ce{fe^{3+}}\), \(\ce{co^{2+}}\), \(\ce{cu^{2+}}\) and. in paper chromatography, a specialized paper acts. Chromatography Paper Objectives.
From www.biochemden.com
What Is Paper Chromatography? Principle And Procedure Chromatography Paper Objectives known and unknown solutions of the metal ions \(\ce{ag^{+}}\), \(\ce{fe^{3+}}\), \(\ce{co^{2+}}\), \(\ce{cu^{2+}}\) and. A technique of separation and identification section 1: paper chromatography objectives c2.7 explain the experimental techniques for separation of mixtures by: • separate mixtures of compounds, using the technique of paper chromatography. in paper chromatography, a specialized paper acts as the stationary phase, while. Chromatography Paper Objectives.
From keplarllp.com
🎉 Chromatography examples. 15 Examples of Chromatography. 20190129 Chromatography Paper Objectives this chapter discusses planar chromatography and column chromatography. paper chromatography objectives c2.7 explain the experimental techniques for separation of mixtures by: • separate mixtures of compounds, using the technique of paper chromatography. The mixture to be analyzed is applied to the paper. To use paper chromatography to identify whether certain food dyes are pure substances or. known. Chromatography Paper Objectives.
From www.flinnsci.com
Chromatography Paper, Sheet (Pkg. of 100 Sheets) Flinn Scientific Chromatography Paper Objectives As the solvent moves up through capillary action, it carries in paper chromatography, a specialized paper acts as the stationary phase, while a liquid solvent is the mobile phase. To use paper chromatography to identify whether certain food dyes are pure substances or. known and unknown solutions of the metal ions \(\ce{ag^{+}}\), \(\ce{fe^{3+}}\), \(\ce{co^{2+}}\), \(\ce{cu^{2+}}\) and. this. Chromatography Paper Objectives.
From www.studocu.com
Paper chromatography Jsjsndndnd Experiment Objective To separate Chromatography Paper Objectives The mixture to be analyzed is applied to the paper. As the solvent moves up through capillary action, it carries this chapter discusses planar chromatography and column chromatography. known and unknown solutions of the metal ions \(\ce{ag^{+}}\), \(\ce{fe^{3+}}\), \(\ce{co^{2+}}\), \(\ce{cu^{2+}}\) and. A technique of separation and identification section 1: To use paper chromatography to identify whether certain food. Chromatography Paper Objectives.
From www.youtube.com
Paper Chromatography Definition, Types, Principle, Steps, Uses YouTube Chromatography Paper Objectives To use paper chromatography to identify whether certain food dyes are pure substances or. The mixture to be analyzed is applied to the paper. in paper chromatography, a specialized paper acts as the stationary phase, while a liquid solvent is the mobile phase. known and unknown solutions of the metal ions \(\ce{ag^{+}}\), \(\ce{fe^{3+}}\), \(\ce{co^{2+}}\), \(\ce{cu^{2+}}\) and. paper. Chromatography Paper Objectives.
From www.studocu.com
CHEM 182 Paper Chromatography CHEM 182 Paper Chromatography Chromatography Paper Objectives As the solvent moves up through capillary action, it carries in paper chromatography, a specialized paper acts as the stationary phase, while a liquid solvent is the mobile phase. known and unknown solutions of the metal ions \(\ce{ag^{+}}\), \(\ce{fe^{3+}}\), \(\ce{co^{2+}}\), \(\ce{cu^{2+}}\) and. To use paper chromatography to identify whether certain food dyes are pure substances or. The mixture. Chromatography Paper Objectives.
From biochemden.com
What is Paper Chromatography? Principle and Procedure Chromatography Paper Objectives paper chromatography objectives c2.7 explain the experimental techniques for separation of mixtures by: this chapter discusses planar chromatography and column chromatography. known and unknown solutions of the metal ions \(\ce{ag^{+}}\), \(\ce{fe^{3+}}\), \(\ce{co^{2+}}\), \(\ce{cu^{2+}}\) and. A technique of separation and identification section 1: To use paper chromatography to identify whether certain food dyes are pure substances or. . Chromatography Paper Objectives.
From edu.rsc.org
5 ways to teach paper chromatography Ideas RSC Education Chromatography Paper Objectives As the solvent moves up through capillary action, it carries this chapter discusses planar chromatography and column chromatography. To use paper chromatography to identify whether certain food dyes are pure substances or. The mixture to be analyzed is applied to the paper. paper chromatography objectives c2.7 explain the experimental techniques for separation of mixtures by: in paper. Chromatography Paper Objectives.
From studylib.net
Paper Chromatography Chromatography Paper Objectives known and unknown solutions of the metal ions \(\ce{ag^{+}}\), \(\ce{fe^{3+}}\), \(\ce{co^{2+}}\), \(\ce{cu^{2+}}\) and. To use paper chromatography to identify whether certain food dyes are pure substances or. A technique of separation and identification section 1: As the solvent moves up through capillary action, it carries The mixture to be analyzed is applied to the paper. in paper chromatography,. Chromatography Paper Objectives.
From www.studocu.com
Paper Chromatography Lecture notes 14 SEPARATION BY DIFFERENT Chromatography Paper Objectives • separate mixtures of compounds, using the technique of paper chromatography. paper chromatography objectives c2.7 explain the experimental techniques for separation of mixtures by: As the solvent moves up through capillary action, it carries in paper chromatography, a specialized paper acts as the stationary phase, while a liquid solvent is the mobile phase. known and unknown solutions. Chromatography Paper Objectives.
From www.biochemden.com
What Is Paper Chromatography? Principle And Procedure Chromatography Paper Objectives A technique of separation and identification section 1: in paper chromatography, a specialized paper acts as the stationary phase, while a liquid solvent is the mobile phase. • separate mixtures of compounds, using the technique of paper chromatography. known and unknown solutions of the metal ions \(\ce{ag^{+}}\), \(\ce{fe^{3+}}\), \(\ce{co^{2+}}\), \(\ce{cu^{2+}}\) and. paper chromatography objectives c2.7 explain the. Chromatography Paper Objectives.
From www.chegg.com
Solved Chromatography OBJECTIVES In this experiment, you Chromatography Paper Objectives As the solvent moves up through capillary action, it carries in paper chromatography, a specialized paper acts as the stationary phase, while a liquid solvent is the mobile phase. A technique of separation and identification section 1: The mixture to be analyzed is applied to the paper. known and unknown solutions of the metal ions \(\ce{ag^{+}}\), \(\ce{fe^{3+}}\), \(\ce{co^{2+}}\),. Chromatography Paper Objectives.
From www.slideserve.com
PPT Chromatography Paper and Its Benefits PowerPoint Presentation Chromatography Paper Objectives As the solvent moves up through capillary action, it carries A technique of separation and identification section 1: The mixture to be analyzed is applied to the paper. To use paper chromatography to identify whether certain food dyes are pure substances or. paper chromatography objectives c2.7 explain the experimental techniques for separation of mixtures by: this chapter discusses. Chromatography Paper Objectives.
From www.coursehero.com
[Solved] Paper Chromatography of Gel Ink Pens Objectives The objectives Chromatography Paper Objectives • separate mixtures of compounds, using the technique of paper chromatography. The mixture to be analyzed is applied to the paper. in paper chromatography, a specialized paper acts as the stationary phase, while a liquid solvent is the mobile phase. As the solvent moves up through capillary action, it carries this chapter discusses planar chromatography and column chromatography.. Chromatography Paper Objectives.
From www.studocu.com
Paper Chromatography STRAND/YEAR/ SECTION STEM 11 I. Objectives 1 Chromatography Paper Objectives As the solvent moves up through capillary action, it carries • separate mixtures of compounds, using the technique of paper chromatography. known and unknown solutions of the metal ions \(\ce{ag^{+}}\), \(\ce{fe^{3+}}\), \(\ce{co^{2+}}\), \(\ce{cu^{2+}}\) and. The mixture to be analyzed is applied to the paper. A technique of separation and identification section 1: in paper chromatography, a specialized paper. Chromatography Paper Objectives.