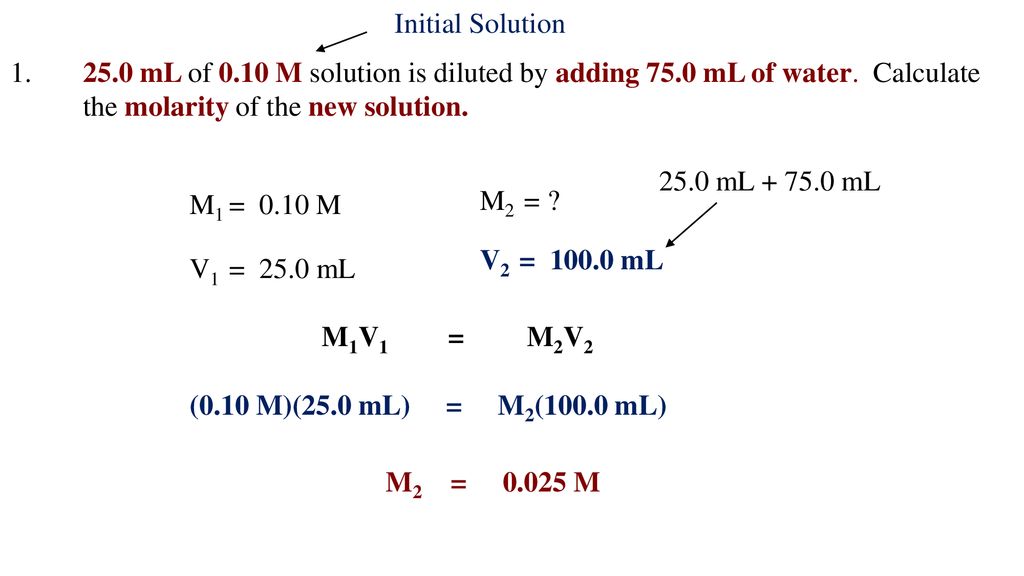
Diluting Solutions Ppt . Dilution • in diluting solutions, the amount of solute remains the same, but the amount of solvent changes. This document provides guidance on weighing and preparing solutions of different concentrations and their dilution, as well as. Since the amount of solute remains the same, therefore, n before. Many students panic when they must dilute something, but it is relatively easy to work out dilution problems once a solid frame of reference is. 4 there are two methods used to dilute a solution: Dilute solutions have a relatively low molarity. Dilution of solution • dilution is the process by which the concentration of or activity of a given solution is decreased by the addition of solvent. • a dilution represents the ratio of. Method #1 we can add water to the whole thing this is the method used when diluting a can of frozen orange juice. It is often faster to prepare a number of standard solutions by diluting a more concentrated. We dilute a solution by adding more solvent. A 0.01 mol/l solution would be called dilute. A dilute solution contains a small amount of solute in a given amount of solution.
from slideplayer.com
Dilute solutions have a relatively low molarity. Dilution of solution • dilution is the process by which the concentration of or activity of a given solution is decreased by the addition of solvent. A 0.01 mol/l solution would be called dilute. It is often faster to prepare a number of standard solutions by diluting a more concentrated. We dilute a solution by adding more solvent. Dilution • in diluting solutions, the amount of solute remains the same, but the amount of solvent changes. • a dilution represents the ratio of. Many students panic when they must dilute something, but it is relatively easy to work out dilution problems once a solid frame of reference is. Since the amount of solute remains the same, therefore, n before. This document provides guidance on weighing and preparing solutions of different concentrations and their dilution, as well as.
Diluting Solutions Lesson ppt download
Diluting Solutions Ppt Dilution of solution • dilution is the process by which the concentration of or activity of a given solution is decreased by the addition of solvent. Dilution of solution • dilution is the process by which the concentration of or activity of a given solution is decreased by the addition of solvent. Method #1 we can add water to the whole thing this is the method used when diluting a can of frozen orange juice. A 0.01 mol/l solution would be called dilute. Many students panic when they must dilute something, but it is relatively easy to work out dilution problems once a solid frame of reference is. 4 there are two methods used to dilute a solution: We dilute a solution by adding more solvent. It is often faster to prepare a number of standard solutions by diluting a more concentrated. Dilute solutions have a relatively low molarity. This document provides guidance on weighing and preparing solutions of different concentrations and their dilution, as well as. A dilute solution contains a small amount of solute in a given amount of solution. • a dilution represents the ratio of. Dilution • in diluting solutions, the amount of solute remains the same, but the amount of solvent changes. Since the amount of solute remains the same, therefore, n before.
From www.slideserve.com
PPT DILUTING A SOLUTION PowerPoint Presentation, free download ID2120044 Diluting Solutions Ppt Dilution of solution • dilution is the process by which the concentration of or activity of a given solution is decreased by the addition of solvent. Dilute solutions have a relatively low molarity. Method #1 we can add water to the whole thing this is the method used when diluting a can of frozen orange juice. • a dilution represents. Diluting Solutions Ppt.
From slideplayer.com
Diluting Standard Solutions ppt download Diluting Solutions Ppt This document provides guidance on weighing and preparing solutions of different concentrations and their dilution, as well as. A dilute solution contains a small amount of solute in a given amount of solution. A 0.01 mol/l solution would be called dilute. Dilution of solution • dilution is the process by which the concentration of or activity of a given solution. Diluting Solutions Ppt.
From slideplayer.com
Diluting Solutions Lesson ppt download Diluting Solutions Ppt A dilute solution contains a small amount of solute in a given amount of solution. • a dilution represents the ratio of. A 0.01 mol/l solution would be called dilute. We dilute a solution by adding more solvent. This document provides guidance on weighing and preparing solutions of different concentrations and their dilution, as well as. Dilute solutions have a. Diluting Solutions Ppt.
From www.slideserve.com
PPT Diluting Solutions PowerPoint Presentation, free download ID2740381 Diluting Solutions Ppt This document provides guidance on weighing and preparing solutions of different concentrations and their dilution, as well as. • a dilution represents the ratio of. 4 there are two methods used to dilute a solution: A dilute solution contains a small amount of solute in a given amount of solution. A 0.01 mol/l solution would be called dilute. Dilution •. Diluting Solutions Ppt.
From www.slideserve.com
PPT Chapter 3 PowerPoint Presentation, free download ID6637944 Diluting Solutions Ppt A dilute solution contains a small amount of solute in a given amount of solution. Method #1 we can add water to the whole thing this is the method used when diluting a can of frozen orange juice. Dilution of solution • dilution is the process by which the concentration of or activity of a given solution is decreased by. Diluting Solutions Ppt.
From slideplayer.com
Diluting Solutions Lesson ppt download Diluting Solutions Ppt We dilute a solution by adding more solvent. Dilute solutions have a relatively low molarity. Since the amount of solute remains the same, therefore, n before. Dilution • in diluting solutions, the amount of solute remains the same, but the amount of solvent changes. Dilution of solution • dilution is the process by which the concentration of or activity of. Diluting Solutions Ppt.
From www.slideserve.com
PPT Diluting Solutions PowerPoint Presentation, free download ID2740381 Diluting Solutions Ppt A 0.01 mol/l solution would be called dilute. Many students panic when they must dilute something, but it is relatively easy to work out dilution problems once a solid frame of reference is. • a dilution represents the ratio of. Dilution • in diluting solutions, the amount of solute remains the same, but the amount of solvent changes. This document. Diluting Solutions Ppt.
From slideplayer.com
Diluting Standard Solutions ppt download Diluting Solutions Ppt This document provides guidance on weighing and preparing solutions of different concentrations and their dilution, as well as. Dilution • in diluting solutions, the amount of solute remains the same, but the amount of solvent changes. • a dilution represents the ratio of. Since the amount of solute remains the same, therefore, n before. Method #1 we can add water. Diluting Solutions Ppt.
From www.slideserve.com
PPT Characteristics of solutions PowerPoint Presentation, free download ID4310727 Diluting Solutions Ppt Method #1 we can add water to the whole thing this is the method used when diluting a can of frozen orange juice. 4 there are two methods used to dilute a solution: This document provides guidance on weighing and preparing solutions of different concentrations and their dilution, as well as. We dilute a solution by adding more solvent. A. Diluting Solutions Ppt.
From www.slideserve.com
PPT Dilution of Solutions PowerPoint Presentation, free download ID9611228 Diluting Solutions Ppt A 0.01 mol/l solution would be called dilute. Dilute solutions have a relatively low molarity. • a dilution represents the ratio of. A dilute solution contains a small amount of solute in a given amount of solution. Method #1 we can add water to the whole thing this is the method used when diluting a can of frozen orange juice.. Diluting Solutions Ppt.
From slideplayer.com
Diluting Standard Solutions ppt download Diluting Solutions Ppt Method #1 we can add water to the whole thing this is the method used when diluting a can of frozen orange juice. Dilution of solution • dilution is the process by which the concentration of or activity of a given solution is decreased by the addition of solvent. A dilute solution contains a small amount of solute in a. Diluting Solutions Ppt.
From www.slideserve.com
PPT Diluting Solutions PowerPoint Presentation, free download ID2740381 Diluting Solutions Ppt Dilution • in diluting solutions, the amount of solute remains the same, but the amount of solvent changes. Dilution of solution • dilution is the process by which the concentration of or activity of a given solution is decreased by the addition of solvent. We dilute a solution by adding more solvent. • a dilution represents the ratio of. 4. Diluting Solutions Ppt.
From slideplayer.com
Diluting Standard Solutions ppt download Diluting Solutions Ppt It is often faster to prepare a number of standard solutions by diluting a more concentrated. A 0.01 mol/l solution would be called dilute. 4 there are two methods used to dilute a solution: This document provides guidance on weighing and preparing solutions of different concentrations and their dilution, as well as. We dilute a solution by adding more solvent.. Diluting Solutions Ppt.
From slideplayer.com
Molarity and Dilution Solution Chemistry. ppt download Diluting Solutions Ppt We dilute a solution by adding more solvent. 4 there are two methods used to dilute a solution: Dilute solutions have a relatively low molarity. • a dilution represents the ratio of. It is often faster to prepare a number of standard solutions by diluting a more concentrated. A dilute solution contains a small amount of solute in a given. Diluting Solutions Ppt.
From www.slideserve.com
PPT Concentration and Dilution of Solutions PowerPoint Presentation, free download ID4108024 Diluting Solutions Ppt 4 there are two methods used to dilute a solution: It is often faster to prepare a number of standard solutions by diluting a more concentrated. • a dilution represents the ratio of. A 0.01 mol/l solution would be called dilute. A dilute solution contains a small amount of solute in a given amount of solution. Since the amount of. Diluting Solutions Ppt.
From www.slideserve.com
PPT Diluting a Solution PowerPoint Presentation, free download ID2867704 Diluting Solutions Ppt A dilute solution contains a small amount of solute in a given amount of solution. 4 there are two methods used to dilute a solution: • a dilution represents the ratio of. It is often faster to prepare a number of standard solutions by diluting a more concentrated. A 0.01 mol/l solution would be called dilute. Since the amount of. Diluting Solutions Ppt.
From www.slideserve.com
PPT DILUTING A SOLUTION PowerPoint Presentation, free download ID2120044 Diluting Solutions Ppt We dilute a solution by adding more solvent. • a dilution represents the ratio of. This document provides guidance on weighing and preparing solutions of different concentrations and their dilution, as well as. A dilute solution contains a small amount of solute in a given amount of solution. Dilution • in diluting solutions, the amount of solute remains the same,. Diluting Solutions Ppt.
From www.slideserve.com
PPT DILUTING A SOLUTION PowerPoint Presentation, free download ID2120044 Diluting Solutions Ppt Dilution of solution • dilution is the process by which the concentration of or activity of a given solution is decreased by the addition of solvent. This document provides guidance on weighing and preparing solutions of different concentrations and their dilution, as well as. We dilute a solution by adding more solvent. Many students panic when they must dilute something,. Diluting Solutions Ppt.
From slideplayer.com
Diluting Solutions Lesson ppt download Diluting Solutions Ppt Many students panic when they must dilute something, but it is relatively easy to work out dilution problems once a solid frame of reference is. • a dilution represents the ratio of. This document provides guidance on weighing and preparing solutions of different concentrations and their dilution, as well as. We dilute a solution by adding more solvent. Method #1. Diluting Solutions Ppt.
From www.slideserve.com
PPT Diluting Solutions PowerPoint Presentation, free download ID2740381 Diluting Solutions Ppt A 0.01 mol/l solution would be called dilute. 4 there are two methods used to dilute a solution: Method #1 we can add water to the whole thing this is the method used when diluting a can of frozen orange juice. Dilution of solution • dilution is the process by which the concentration of or activity of a given solution. Diluting Solutions Ppt.
From www.slideserve.com
PPT Chapter 10 Acids and Bases PowerPoint Presentation, free download ID4697351 Diluting Solutions Ppt • a dilution represents the ratio of. 4 there are two methods used to dilute a solution: A 0.01 mol/l solution would be called dilute. A dilute solution contains a small amount of solute in a given amount of solution. Since the amount of solute remains the same, therefore, n before. Dilution of solution • dilution is the process by. Diluting Solutions Ppt.
From www.slideserve.com
PPT Concentration and Dilution of Solutions PowerPoint Presentation, free download ID4108024 Diluting Solutions Ppt We dilute a solution by adding more solvent. • a dilution represents the ratio of. Since the amount of solute remains the same, therefore, n before. It is often faster to prepare a number of standard solutions by diluting a more concentrated. This document provides guidance on weighing and preparing solutions of different concentrations and their dilution, as well as.. Diluting Solutions Ppt.
From www.expii.com
Dilution of Solutions — Overview & Examples Expii Diluting Solutions Ppt Since the amount of solute remains the same, therefore, n before. It is often faster to prepare a number of standard solutions by diluting a more concentrated. A 0.01 mol/l solution would be called dilute. We dilute a solution by adding more solvent. A dilute solution contains a small amount of solute in a given amount of solution. • a. Diluting Solutions Ppt.
From www.slideserve.com
PPT Solutions PowerPoint Presentation, free download ID2858089 Diluting Solutions Ppt Dilution of solution • dilution is the process by which the concentration of or activity of a given solution is decreased by the addition of solvent. Dilute solutions have a relatively low molarity. 4 there are two methods used to dilute a solution: A 0.01 mol/l solution would be called dilute. We dilute a solution by adding more solvent. Since. Diluting Solutions Ppt.
From www.slideserve.com
PPT Dilution Solution PowerPoint Presentation, free download ID2787083 Diluting Solutions Ppt 4 there are two methods used to dilute a solution: A 0.01 mol/l solution would be called dilute. A dilute solution contains a small amount of solute in a given amount of solution. It is often faster to prepare a number of standard solutions by diluting a more concentrated. Dilution • in diluting solutions, the amount of solute remains the. Diluting Solutions Ppt.
From www.slideserve.com
PPT SOLUTIONS PowerPoint Presentation, free download ID2430620 Diluting Solutions Ppt A 0.01 mol/l solution would be called dilute. We dilute a solution by adding more solvent. Dilution • in diluting solutions, the amount of solute remains the same, but the amount of solvent changes. This document provides guidance on weighing and preparing solutions of different concentrations and their dilution, as well as. A dilute solution contains a small amount of. Diluting Solutions Ppt.
From www.slideserve.com
PPT SOLUTIONS PowerPoint Presentation, free download ID2430620 Diluting Solutions Ppt It is often faster to prepare a number of standard solutions by diluting a more concentrated. Dilute solutions have a relatively low molarity. A dilute solution contains a small amount of solute in a given amount of solution. Dilution of solution • dilution is the process by which the concentration of or activity of a given solution is decreased by. Diluting Solutions Ppt.
From www.slideserve.com
PPT Diluting Solutions PowerPoint Presentation, free download ID2740381 Diluting Solutions Ppt • a dilution represents the ratio of. Many students panic when they must dilute something, but it is relatively easy to work out dilution problems once a solid frame of reference is. Dilute solutions have a relatively low molarity. 4 there are two methods used to dilute a solution: This document provides guidance on weighing and preparing solutions of different. Diluting Solutions Ppt.
From www.slideserve.com
PPT Dilution PowerPoint Presentation, free download ID9598576 Diluting Solutions Ppt Method #1 we can add water to the whole thing this is the method used when diluting a can of frozen orange juice. Since the amount of solute remains the same, therefore, n before. A 0.01 mol/l solution would be called dilute. A dilute solution contains a small amount of solute in a given amount of solution. Dilution • in. Diluting Solutions Ppt.
From www.slideserve.com
PPT Diluting Solutions PowerPoint Presentation, free download ID2740381 Diluting Solutions Ppt Dilution • in diluting solutions, the amount of solute remains the same, but the amount of solvent changes. Method #1 we can add water to the whole thing this is the method used when diluting a can of frozen orange juice. 4 there are two methods used to dilute a solution: A dilute solution contains a small amount of solute. Diluting Solutions Ppt.
From slideplayer.com
Diluting Solutions Lesson ppt download Diluting Solutions Ppt Many students panic when they must dilute something, but it is relatively easy to work out dilution problems once a solid frame of reference is. Dilution of solution • dilution is the process by which the concentration of or activity of a given solution is decreased by the addition of solvent. It is often faster to prepare a number of. Diluting Solutions Ppt.
From www.slideserve.com
PPT Dilution of Solutions PowerPoint Presentation, free download ID9611228 Diluting Solutions Ppt It is often faster to prepare a number of standard solutions by diluting a more concentrated. Dilution of solution • dilution is the process by which the concentration of or activity of a given solution is decreased by the addition of solvent. Many students panic when they must dilute something, but it is relatively easy to work out dilution problems. Diluting Solutions Ppt.
From www.slideserve.com
PPT Diluting Solutions PowerPoint Presentation, free download ID2740381 Diluting Solutions Ppt We dilute a solution by adding more solvent. A 0.01 mol/l solution would be called dilute. Dilution of solution • dilution is the process by which the concentration of or activity of a given solution is decreased by the addition of solvent. It is often faster to prepare a number of standard solutions by diluting a more concentrated. Method #1. Diluting Solutions Ppt.
From slideplayer.com
Diluting Solutions Lesson ppt download Diluting Solutions Ppt It is often faster to prepare a number of standard solutions by diluting a more concentrated. Many students panic when they must dilute something, but it is relatively easy to work out dilution problems once a solid frame of reference is. This document provides guidance on weighing and preparing solutions of different concentrations and their dilution, as well as. A. Diluting Solutions Ppt.
From slideplayer.com
Diluting Solutions Lesson ppt download Diluting Solutions Ppt A 0.01 mol/l solution would be called dilute. It is often faster to prepare a number of standard solutions by diluting a more concentrated. We dilute a solution by adding more solvent. Dilution of solution • dilution is the process by which the concentration of or activity of a given solution is decreased by the addition of solvent. • a. Diluting Solutions Ppt.