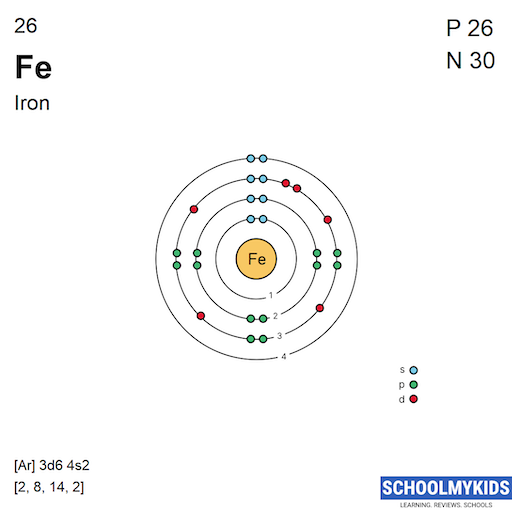
Iron Ground State Electron Configuration . Its core orbitals are the #1s#, #2s#, #2p# 's, #3s#, and #3p# 's. Identify and explain exceptions to predicted electron configurations for atoms and ions. Its valence orbitals are the #4s# and #3d# 's. From the electrons in an atom, to the. Writing the electron configuration, you really only. The shorthand electron configuration (or noble gas configuration) as well as. Ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. This electron configuration shows that the last shell of iron has two electrons and the d. Electron configuration chart of all elements is mentioned in the table below. In order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there are 26 electrons). The ground state electron configuration of iron is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 6 4s 2. Identify and explain exceptions to predicted electron configurations.
from www.schoolmykids.com
This electron configuration shows that the last shell of iron has two electrons and the d. The ground state electron configuration of iron is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 6 4s 2. From the electrons in an atom, to the. In order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there are 26 electrons). Identify and explain exceptions to predicted electron configurations. Writing the electron configuration, you really only. The shorthand electron configuration (or noble gas configuration) as well as. Its core orbitals are the #1s#, #2s#, #2p# 's, #3s#, and #3p# 's. Identify and explain exceptions to predicted electron configurations for atoms and ions. Electron configuration chart of all elements is mentioned in the table below.
Iron (Fe) Element Information, Facts, Properties, Uses Periodic Table of the Elements
Iron Ground State Electron Configuration Its core orbitals are the #1s#, #2s#, #2p# 's, #3s#, and #3p# 's. In order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there are 26 electrons). The ground state electron configuration of iron is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 6 4s 2. This electron configuration shows that the last shell of iron has two electrons and the d. Writing the electron configuration, you really only. Ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. Its valence orbitals are the #4s# and #3d# 's. From the electrons in an atom, to the. Its core orbitals are the #1s#, #2s#, #2p# 's, #3s#, and #3p# 's. Electron configuration chart of all elements is mentioned in the table below. The shorthand electron configuration (or noble gas configuration) as well as. Identify and explain exceptions to predicted electron configurations. Identify and explain exceptions to predicted electron configurations for atoms and ions.
From general.chemistrysteps.com
Electron Configurations Chemistry Steps Iron Ground State Electron Configuration Identify and explain exceptions to predicted electron configurations. Its valence orbitals are the #4s# and #3d# 's. Electron configuration chart of all elements is mentioned in the table below. Ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. The ground state electron configuration of iron is 1s 2 2s 2 2p 6 3s 2 3p. Iron Ground State Electron Configuration.
From www.researchgate.net
Electronic configurations energy levels of the ground state 3 O 2... Download Scientific Diagram Iron Ground State Electron Configuration Writing the electron configuration, you really only. The shorthand electron configuration (or noble gas configuration) as well as. Identify and explain exceptions to predicted electron configurations. From the electrons in an atom, to the. In order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there are 26 electrons). Its. Iron Ground State Electron Configuration.
From chem.libretexts.org
Electronic Configurations Intro Chemistry LibreTexts Iron Ground State Electron Configuration Ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. Identify and explain exceptions to predicted electron configurations. Identify and explain exceptions to predicted electron configurations for atoms and ions. Writing the electron configuration, you really only. Its valence orbitals are the #4s# and #3d# 's. The ground state electron configuration of iron is 1s 2. Iron Ground State Electron Configuration.
From www.youtube.com
How To Determine The Number of Paired and Unpaired Electrons YouTube Iron Ground State Electron Configuration Its valence orbitals are the #4s# and #3d# 's. In order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there are 26 electrons). Its core orbitals are the #1s#, #2s#, #2p# 's, #3s#, and #3p# 's. This electron configuration shows that the last shell of iron has two electrons. Iron Ground State Electron Configuration.
From brandonkss.github.io
Ground State Electron Configuration Chart Iron Ground State Electron Configuration Its core orbitals are the #1s#, #2s#, #2p# 's, #3s#, and #3p# 's. Writing the electron configuration, you really only. Identify and explain exceptions to predicted electron configurations for atoms and ions. Identify and explain exceptions to predicted electron configurations. The ground state electron configuration of iron is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 6. Iron Ground State Electron Configuration.
From learnwithdrscott.com
Electron Configuration Worksheet Easy Hard Science Iron Ground State Electron Configuration The ground state electron configuration of iron is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 6 4s 2. Its valence orbitals are the #4s# and #3d# 's. Identify and explain exceptions to predicted electron configurations. Identify and explain exceptions to predicted electron configurations for atoms and ions. From the electrons in an atom, to the. In. Iron Ground State Electron Configuration.
From quizlet.com
Draw the electron configuration for a neutral atom of iron. Quizlet Iron Ground State Electron Configuration Electron configuration chart of all elements is mentioned in the table below. Identify and explain exceptions to predicted electron configurations for atoms and ions. Ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. This electron configuration shows that the last shell of iron has two electrons and the d. The shorthand electron configuration (or noble. Iron Ground State Electron Configuration.
From general.chemistrysteps.com
Electron Configurations of Ions Chemistry Steps Iron Ground State Electron Configuration Ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. This electron configuration shows that the last shell of iron has two electrons and the d. Its core orbitals are the #1s#, #2s#, #2p# 's, #3s#, and #3p# 's. The shorthand electron configuration (or noble gas configuration) as well as. Electron configuration chart of all elements. Iron Ground State Electron Configuration.
From chem.libretexts.org
Chapter 2.7 Electronic Structure of the Transition Metals Chemistry LibreTexts Iron Ground State Electron Configuration Identify and explain exceptions to predicted electron configurations. From the electrons in an atom, to the. Writing the electron configuration, you really only. The shorthand electron configuration (or noble gas configuration) as well as. Electron configuration chart of all elements is mentioned in the table below. Its core orbitals are the #1s#, #2s#, #2p# 's, #3s#, and #3p# 's. Ground. Iron Ground State Electron Configuration.
From www.youtube.com
Iron electronic configuration How to Write Iron electronic configuration YouTube Iron Ground State Electron Configuration The shorthand electron configuration (or noble gas configuration) as well as. Its core orbitals are the #1s#, #2s#, #2p# 's, #3s#, and #3p# 's. Writing the electron configuration, you really only. From the electrons in an atom, to the. Electron configuration chart of all elements is mentioned in the table below. This electron configuration shows that the last shell of. Iron Ground State Electron Configuration.
From newlasertagatlanta.blogspot.com
42 fe2+ orbital diagram Wiring Diagrams Manual Iron Ground State Electron Configuration Identify and explain exceptions to predicted electron configurations. Electron configuration chart of all elements is mentioned in the table below. In order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there are 26 electrons). The shorthand electron configuration (or noble gas configuration) as well as. Its valence orbitals are. Iron Ground State Electron Configuration.
From general.chemistrysteps.com
Electron Configurations of Ions Chemistry Steps Iron Ground State Electron Configuration From the electrons in an atom, to the. Ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. Its valence orbitals are the #4s# and #3d# 's. Writing the electron configuration, you really only. In order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there. Iron Ground State Electron Configuration.
From ar.inspiredpencil.com
Iron Orbital Notation Iron Ground State Electron Configuration The shorthand electron configuration (or noble gas configuration) as well as. The ground state electron configuration of iron is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 6 4s 2. In order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there are 26 electrons). From the. Iron Ground State Electron Configuration.
From brandonkss.github.io
Ground State Electron Configuration Chart Iron Ground State Electron Configuration Its valence orbitals are the #4s# and #3d# 's. Writing the electron configuration, you really only. The shorthand electron configuration (or noble gas configuration) as well as. Identify and explain exceptions to predicted electron configurations. Electron configuration chart of all elements is mentioned in the table below. Its core orbitals are the #1s#, #2s#, #2p# 's, #3s#, and #3p# 's.. Iron Ground State Electron Configuration.
From www.youtube.com
Electron Configuration for Fe, Fe2+, and Fe3+ (Iron and Iron Ions) YouTube Iron Ground State Electron Configuration Writing the electron configuration, you really only. The shorthand electron configuration (or noble gas configuration) as well as. Electron configuration chart of all elements is mentioned in the table below. From the electrons in an atom, to the. Its valence orbitals are the #4s# and #3d# 's. This electron configuration shows that the last shell of iron has two electrons. Iron Ground State Electron Configuration.
From socratic.org
Write the ground state electron configuration for a neutral carbon atom, and for an excited Iron Ground State Electron Configuration In order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there are 26 electrons). Its valence orbitals are the #4s# and #3d# 's. The shorthand electron configuration (or noble gas configuration) as well as. Writing the electron configuration, you really only. Identify and explain exceptions to predicted electron configurations. Iron Ground State Electron Configuration.
From www.webelements.com
Elements Periodic Table » Iron » properties of free atoms Iron Ground State Electron Configuration Electron configuration chart of all elements is mentioned in the table below. This electron configuration shows that the last shell of iron has two electrons and the d. The shorthand electron configuration (or noble gas configuration) as well as. In order to write the iron electron configuration we first need to know the number of electrons for the fe atom. Iron Ground State Electron Configuration.
From avopix.com
Electron configurations of the ground States of Royalty Free Stock Vector 1477039154 Iron Ground State Electron Configuration The shorthand electron configuration (or noble gas configuration) as well as. Its core orbitals are the #1s#, #2s#, #2p# 's, #3s#, and #3p# 's. Identify and explain exceptions to predicted electron configurations for atoms and ions. Electron configuration chart of all elements is mentioned in the table below. The ground state electron configuration of iron is 1s 2 2s 2. Iron Ground State Electron Configuration.
From valenceelectrons.com
Electron Configuration for Iron (Fe and Fe2+, Fe3+ ions) Iron Ground State Electron Configuration In order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there are 26 electrons). Ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. The ground state electron configuration of iron is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 6 4s. Iron Ground State Electron Configuration.
From firsteducationinfo.com
Ground State Electron Configuration First Education Info Iron Ground State Electron Configuration In order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there are 26 electrons). The ground state electron configuration of iron is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 6 4s 2. From the electrons in an atom, to the. Writing the electron configuration, you. Iron Ground State Electron Configuration.
From mungfali.com
Ground State Electron Configuration Chart Iron Ground State Electron Configuration The shorthand electron configuration (or noble gas configuration) as well as. Ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. The ground state electron configuration of iron is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 6 4s 2. Its core orbitals are the #1s#, #2s#, #2p# 's, #3s#, and #3p# 's.. Iron Ground State Electron Configuration.
From www.orangatame.com
How to Write Ground State Electron Configuration in Chemistry Iron Ground State Electron Configuration From the electrons in an atom, to the. In order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there are 26 electrons). Electron configuration chart of all elements is mentioned in the table below. The ground state electron configuration of iron is 1s 2 2s 2 2p 6 3s. Iron Ground State Electron Configuration.
From www.numerade.com
SOLVEDWrite the groundstate electron configurations for the following elements Ge, Fe, Zn, Ni Iron Ground State Electron Configuration From the electrons in an atom, to the. Identify and explain exceptions to predicted electron configurations for atoms and ions. The ground state electron configuration of iron is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 6 4s 2. This electron configuration shows that the last shell of iron has two electrons and the d. Writing the. Iron Ground State Electron Configuration.
From mavink.com
Ground State Electron Configuration Chart Iron Ground State Electron Configuration The shorthand electron configuration (or noble gas configuration) as well as. Its valence orbitals are the #4s# and #3d# 's. In order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there are 26 electrons). This electron configuration shows that the last shell of iron has two electrons and the. Iron Ground State Electron Configuration.
From www.sciencefacts.net
Electron Configuration Definition, Examples, Chart, and Diagram Iron Ground State Electron Configuration Ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. Identify and explain exceptions to predicted electron configurations for atoms and ions. The shorthand electron configuration (or noble gas configuration) as well as. Writing the electron configuration, you really only. Its valence orbitals are the #4s# and #3d# 's. From the electrons in an atom, to. Iron Ground State Electron Configuration.
From wisc.pb.unizin.org
Chapter 3 Electron Configurations and the Periodic Table Chemistry 109 Iron Ground State Electron Configuration In order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there are 26 electrons). Identify and explain exceptions to predicted electron configurations. Writing the electron configuration, you really only. The shorthand electron configuration (or noble gas configuration) as well as. Identify and explain exceptions to predicted electron configurations for. Iron Ground State Electron Configuration.
From denkerensterly.blogspot.com
Predict the Ground State Electron Configuration Co3+ Denker Ensterly Iron Ground State Electron Configuration Its core orbitals are the #1s#, #2s#, #2p# 's, #3s#, and #3p# 's. From the electrons in an atom, to the. The ground state electron configuration of iron is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 6 4s 2. Ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. Writing the electron. Iron Ground State Electron Configuration.
From www.orangatame.com
How to Write Ground State Electron Configuration in Chemistry Iron Ground State Electron Configuration Writing the electron configuration, you really only. From the electrons in an atom, to the. The shorthand electron configuration (or noble gas configuration) as well as. The ground state electron configuration of iron is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 6 4s 2. Identify and explain exceptions to predicted electron configurations. Ground state electron configurations. Iron Ground State Electron Configuration.
From wisc.pb.unizin.org
Electron Configurations, Orbital Box Notation (M7Q7) UWMadison Chemistry 103/104 Resource Book Iron Ground State Electron Configuration Writing the electron configuration, you really only. This electron configuration shows that the last shell of iron has two electrons and the d. Identify and explain exceptions to predicted electron configurations for atoms and ions. The ground state electron configuration of iron is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 6 4s 2. Electron configuration chart. Iron Ground State Electron Configuration.
From www.numerade.com
SOLVED Write the groundstate electron configuration for a neutral atom of each element iron Iron Ground State Electron Configuration Its valence orbitals are the #4s# and #3d# 's. In order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there are 26 electrons). Writing the electron configuration, you really only. This electron configuration shows that the last shell of iron has two electrons and the d. From the electrons. Iron Ground State Electron Configuration.
From commons.wikimedia.org
Original file (SVG file, nominally 334 × 254 pixels, file size 42 KB) Iron Ground State Electron Configuration Its valence orbitals are the #4s# and #3d# 's. Its core orbitals are the #1s#, #2s#, #2p# 's, #3s#, and #3p# 's. Electron configuration chart of all elements is mentioned in the table below. In order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there are 26 electrons). The. Iron Ground State Electron Configuration.
From www.schoolmykids.com
Iron (Fe) Element Information, Facts, Properties, Uses Periodic Table of the Elements Iron Ground State Electron Configuration Identify and explain exceptions to predicted electron configurations for atoms and ions. The shorthand electron configuration (or noble gas configuration) as well as. The ground state electron configuration of iron is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 6 4s 2. Identify and explain exceptions to predicted electron configurations. In order to write the iron electron. Iron Ground State Electron Configuration.
From www.orangatame.com
How to Write Ground State Electron Configuration in Chemistry Iron Ground State Electron Configuration From the electrons in an atom, to the. Ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. Writing the electron configuration, you really only. In order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there are 26 electrons). Identify and explain exceptions to predicted. Iron Ground State Electron Configuration.
From learnwithdrscott.com
Electron Configuration Worksheet Easy Hard Science Iron Ground State Electron Configuration The shorthand electron configuration (or noble gas configuration) as well as. The ground state electron configuration of iron is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 6 4s 2. Writing the electron configuration, you really only. Identify and explain exceptions to predicted electron configurations for atoms and ions. From the electrons in an atom, to the.. Iron Ground State Electron Configuration.
From sciencenotes.org
List of Electron Configurations of Elements Iron Ground State Electron Configuration Ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. Its valence orbitals are the #4s# and #3d# 's. The shorthand electron configuration (or noble gas configuration) as well as. Identify and explain exceptions to predicted electron configurations for atoms and ions. From the electrons in an atom, to the. Its core orbitals are the #1s#,. Iron Ground State Electron Configuration.