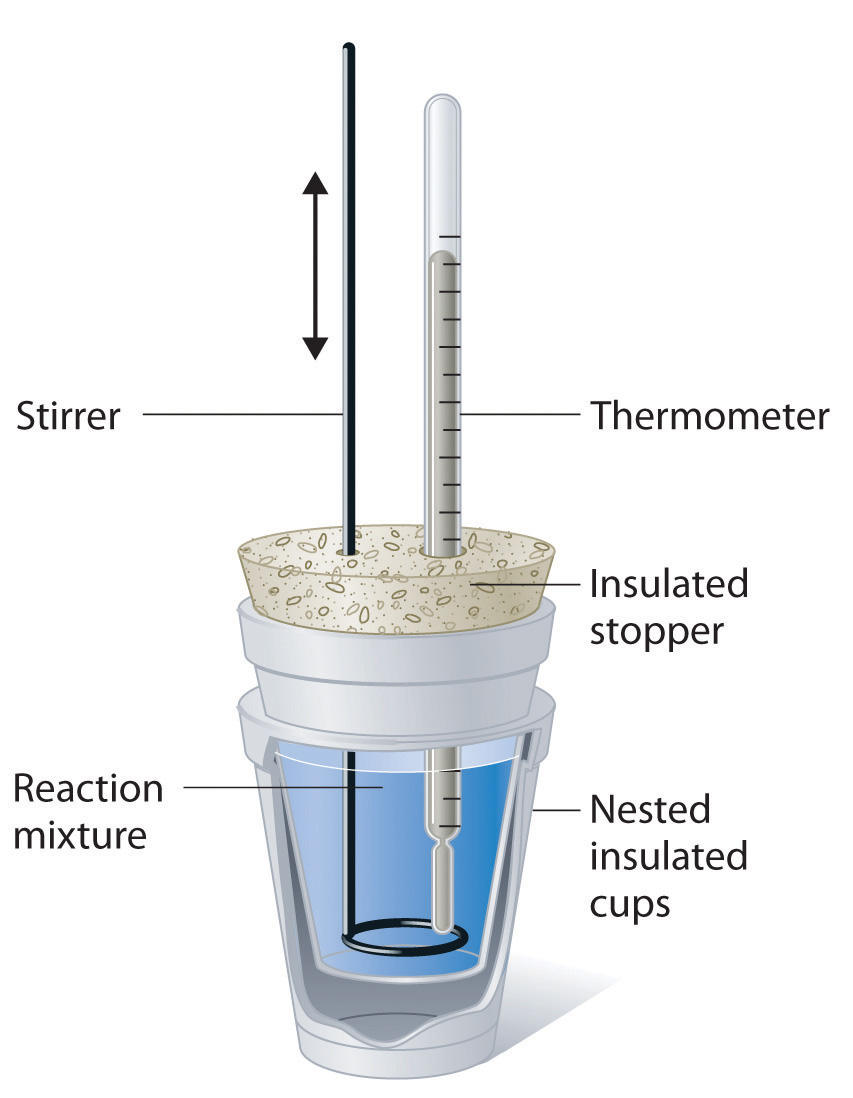
Calorimeter How To Find Temperature . We will look at two ways of measuring this heat, either the temperature change of the calorimeter, or the mass of a substance that undergoes a phase change within the. \ [q = mc\delta t,\] where \ (q\) is the symbol for heat transfer (“quantity of heat”), m. Calorimetry is used to measure amounts of heat transferred to or from a substance. We need to find the difference between the heat lost by the hot water when it droped from 60.0 to 40.0 and the heat gained by the cold water. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of heat released or absorbed and. A practical approximation for the relationship between heat transfer and temperature change is: Calorimetry is a science where you try to find. Read on to learn what calorimetry is and how to solve calorimetry problems with the proper equations. To do so, the heat is exchanged with a calibrated object. The temperature change produced by the known reaction is used to determine the heat capacity of the calorimeter.
from 2012books.lardbucket.org
\ [q = mc\delta t,\] where \ (q\) is the symbol for heat transfer (“quantity of heat”), m. To do so, the heat is exchanged with a calibrated object. A practical approximation for the relationship between heat transfer and temperature change is: Read on to learn what calorimetry is and how to solve calorimetry problems with the proper equations. Calorimetry is a science where you try to find. Calorimetry is used to measure amounts of heat transferred to or from a substance. We will look at two ways of measuring this heat, either the temperature change of the calorimeter, or the mass of a substance that undergoes a phase change within the. We need to find the difference between the heat lost by the hot water when it droped from 60.0 to 40.0 and the heat gained by the cold water. The temperature change produced by the known reaction is used to determine the heat capacity of the calorimeter. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of heat released or absorbed and.
Calorimetry
Calorimeter How To Find Temperature Calorimetry is used to measure amounts of heat transferred to or from a substance. \ [q = mc\delta t,\] where \ (q\) is the symbol for heat transfer (“quantity of heat”), m. Calorimetry is used to measure amounts of heat transferred to or from a substance. We will look at two ways of measuring this heat, either the temperature change of the calorimeter, or the mass of a substance that undergoes a phase change within the. Calorimetry is a science where you try to find. To do so, the heat is exchanged with a calibrated object. We need to find the difference between the heat lost by the hot water when it droped from 60.0 to 40.0 and the heat gained by the cold water. A practical approximation for the relationship between heat transfer and temperature change is: The temperature change produced by the known reaction is used to determine the heat capacity of the calorimeter. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of heat released or absorbed and. Read on to learn what calorimetry is and how to solve calorimetry problems with the proper equations.
From www.youtube.com
Temperature and Heat Calorimetry. Level 2, Example 2 YouTube Calorimeter How To Find Temperature We will look at two ways of measuring this heat, either the temperature change of the calorimeter, or the mass of a substance that undergoes a phase change within the. Read on to learn what calorimetry is and how to solve calorimetry problems with the proper equations. A practical approximation for the relationship between heat transfer and temperature change is:. Calorimeter How To Find Temperature.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID1875569 Calorimeter How To Find Temperature Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of heat released or absorbed and. Read on to learn what calorimetry is and how to solve calorimetry problems with the proper equations. To do so, the heat. Calorimeter How To Find Temperature.
From printablezonemarrow.z13.web.core.windows.net
How To Calculate Heat Of Reaction Calorimetry Calorimeter How To Find Temperature Calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a calibrated object. A practical approximation for the relationship between heat transfer and temperature change is: \ [q = mc\delta t,\] where \ (q\) is the symbol for heat transfer (“quantity of heat”), m. Read on to learn. Calorimeter How To Find Temperature.
From www.youtube.com
CHEMISTRY 101 Calculating Heat Capacity of a Bomb Calorimeter YouTube Calorimeter How To Find Temperature The temperature change produced by the known reaction is used to determine the heat capacity of the calorimeter. Calorimetry is used to measure amounts of heat transferred to or from a substance. We will look at two ways of measuring this heat, either the temperature change of the calorimeter, or the mass of a substance that undergoes a phase change. Calorimeter How To Find Temperature.
From chemwiki.ucdavis.edu
Chapter 9.6 Calorimetry Chemwiki Calorimeter How To Find Temperature Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of heat released or absorbed and. We need to find the difference between the heat lost by the hot water when it droped from 60.0 to 40.0 and. Calorimeter How To Find Temperature.
From deon-has-edwards.blogspot.com
Heat Capacity of Calorimeter DeonhasEdwards Calorimeter How To Find Temperature We will look at two ways of measuring this heat, either the temperature change of the calorimeter, or the mass of a substance that undergoes a phase change within the. Calorimetry is a science where you try to find. To do so, the heat is exchanged with a calibrated object. Calorimetry is used to measure amounts of heat transferred to. Calorimeter How To Find Temperature.
From quizdbbarefooted.z21.web.core.windows.net
How To Calculate Calorimeter Calorimeter How To Find Temperature Calorimetry is a science where you try to find. Read on to learn what calorimetry is and how to solve calorimetry problems with the proper equations. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of heat released or absorbed and. We will look at two ways of measuring this heat,. Calorimeter How To Find Temperature.
From quiztricksters.z21.web.core.windows.net
How To Calculate Calorimeter Calorimeter How To Find Temperature A practical approximation for the relationship between heat transfer and temperature change is: Calorimetry is a science where you try to find. We need to find the difference between the heat lost by the hot water when it droped from 60.0 to 40.0 and the heat gained by the cold water. To do so, the heat is exchanged with a. Calorimeter How To Find Temperature.
From quizmischances.z4.web.core.windows.net
How To Calculate Calorimetry Calorimeter How To Find Temperature Calorimetry is a science where you try to find. The temperature change produced by the known reaction is used to determine the heat capacity of the calorimeter. \ [q = mc\delta t,\] where \ (q\) is the symbol for heat transfer (“quantity of heat”), m. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. Calorimeter How To Find Temperature.
From www.youtube.com
Thermochemistry 5 Determining Calorimeter Constant 6m16s YouTube Calorimeter How To Find Temperature Calorimetry is a science where you try to find. The temperature change produced by the known reaction is used to determine the heat capacity of the calorimeter. Calorimetry is used to measure amounts of heat transferred to or from a substance. We need to find the difference between the heat lost by the hot water when it droped from 60.0. Calorimeter How To Find Temperature.
From haipernews.com
How To Calculate Heat Capacity From Calorimeter Haiper Calorimeter How To Find Temperature Calorimetry is a science where you try to find. \ [q = mc\delta t,\] where \ (q\) is the symbol for heat transfer (“quantity of heat”), m. We need to find the difference between the heat lost by the hot water when it droped from 60.0 to 40.0 and the heat gained by the cold water. The temperature change produced. Calorimeter How To Find Temperature.
From learningcampusscarf.z13.web.core.windows.net
How To Calculate Heat Of Reaction Calorimetry Calorimeter How To Find Temperature Calorimetry is used to measure amounts of heat transferred to or from a substance. The temperature change produced by the known reaction is used to determine the heat capacity of the calorimeter. Calorimetry is a science where you try to find. We will look at two ways of measuring this heat, either the temperature change of the calorimeter, or the. Calorimeter How To Find Temperature.
From www.youtube.com
Thermal Properties of Matter Class 11 Physics Calorimetry Principle Calorimeter How To Find Temperature Calorimetry is a science where you try to find. We need to find the difference between the heat lost by the hot water when it droped from 60.0 to 40.0 and the heat gained by the cold water. To do so, the heat is exchanged with a calibrated object. We will look at two ways of measuring this heat, either. Calorimeter How To Find Temperature.
From 2012books.lardbucket.org
Calorimetry Calorimeter How To Find Temperature Calorimetry is used to measure amounts of heat transferred to or from a substance. Read on to learn what calorimetry is and how to solve calorimetry problems with the proper equations. Calorimetry is a science where you try to find. The temperature change produced by the known reaction is used to determine the heat capacity of the calorimeter. To do. Calorimeter How To Find Temperature.
From www.slideserve.com
PPT Unit 13 Thermochemistry PowerPoint Presentation ID6591088 Calorimeter How To Find Temperature A practical approximation for the relationship between heat transfer and temperature change is: We will look at two ways of measuring this heat, either the temperature change of the calorimeter, or the mass of a substance that undergoes a phase change within the. Read on to learn what calorimetry is and how to solve calorimetry problems with the proper equations.. Calorimeter How To Find Temperature.
From www.youtube.com
Final Temperature Calorimetry Practice Problems Chemistry YouTube Calorimeter How To Find Temperature A practical approximation for the relationship between heat transfer and temperature change is: Read on to learn what calorimetry is and how to solve calorimetry problems with the proper equations. \ [q = mc\delta t,\] where \ (q\) is the symbol for heat transfer (“quantity of heat”), m. To do so, the heat is exchanged with a calibrated object. Calorimetry. Calorimeter How To Find Temperature.
From www.showme.com
4 steps for solving calorimetry problems Science, Chemistry ShowMe Calorimeter How To Find Temperature To do so, the heat is exchanged with a calibrated object. A practical approximation for the relationship between heat transfer and temperature change is: We need to find the difference between the heat lost by the hot water when it droped from 60.0 to 40.0 and the heat gained by the cold water. We will look at two ways of. Calorimeter How To Find Temperature.
From quiztricksters.z21.web.core.windows.net
How To Calculate Calorimeter Calorimeter How To Find Temperature Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of heat released or absorbed and. Calorimetry is a science where you try to find. To do so, the heat is exchanged with a calibrated object. Calorimetry is used to measure amounts of heat transferred to or from a substance. The temperature. Calorimeter How To Find Temperature.
From quiztricksters.z21.web.core.windows.net
How To Calculate Calorimeter Calorimeter How To Find Temperature To do so, the heat is exchanged with a calibrated object. Calorimetry is used to measure amounts of heat transferred to or from a substance. Read on to learn what calorimetry is and how to solve calorimetry problems with the proper equations. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount. Calorimeter How To Find Temperature.
From www.youtube.com
AP Chemistry Thermochemical Equations and Calorimetry YouTube Calorimeter How To Find Temperature \ [q = mc\delta t,\] where \ (q\) is the symbol for heat transfer (“quantity of heat”), m. We need to find the difference between the heat lost by the hot water when it droped from 60.0 to 40.0 and the heat gained by the cold water. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature. Calorimeter How To Find Temperature.
From www.animalia-life.club
Calorimeter Diagram Calorimeter How To Find Temperature Read on to learn what calorimetry is and how to solve calorimetry problems with the proper equations. We will look at two ways of measuring this heat, either the temperature change of the calorimeter, or the mass of a substance that undergoes a phase change within the. A practical approximation for the relationship between heat transfer and temperature change is:. Calorimeter How To Find Temperature.
From chem.libretexts.org
12 Calorimetry and Hess's Law (Experiment) Chemistry LibreTexts Calorimeter How To Find Temperature Calorimetry is used to measure amounts of heat transferred to or from a substance. We need to find the difference between the heat lost by the hot water when it droped from 60.0 to 40.0 and the heat gained by the cold water. The temperature change produced by the known reaction is used to determine the heat capacity of the. Calorimeter How To Find Temperature.
From www.scienceabc.com
Molar Heat Capacity Definition, Formula, Equation, Calculation Calorimeter How To Find Temperature The temperature change produced by the known reaction is used to determine the heat capacity of the calorimeter. We need to find the difference between the heat lost by the hot water when it droped from 60.0 to 40.0 and the heat gained by the cold water. We will look at two ways of measuring this heat, either the temperature. Calorimeter How To Find Temperature.
From fyohqoqgp.blob.core.windows.net
Calorimeter Formula In Physics at Caroline Graig blog Calorimeter How To Find Temperature Calorimetry is a science where you try to find. \ [q = mc\delta t,\] where \ (q\) is the symbol for heat transfer (“quantity of heat”), m. We will look at two ways of measuring this heat, either the temperature change of the calorimeter, or the mass of a substance that undergoes a phase change within the. The temperature change. Calorimeter How To Find Temperature.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica Calorimeter How To Find Temperature We will look at two ways of measuring this heat, either the temperature change of the calorimeter, or the mass of a substance that undergoes a phase change within the. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on. Calorimeter How To Find Temperature.
From www.sliderbase.com
Basic Thermochemistry Presentation Chemistry Calorimeter How To Find Temperature Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of heat released or absorbed and. The temperature change produced by the known reaction is used to determine the heat capacity of the calorimeter. We need to find the difference between the heat lost by the hot water when it droped from. Calorimeter How To Find Temperature.
From courses.lumenlearning.com
Calorimetry Chemistry I Calorimeter How To Find Temperature \ [q = mc\delta t,\] where \ (q\) is the symbol for heat transfer (“quantity of heat”), m. We need to find the difference between the heat lost by the hot water when it droped from 60.0 to 40.0 and the heat gained by the cold water. The temperature change produced by the known reaction is used to determine the. Calorimeter How To Find Temperature.
From www.slideserve.com
PPT Introduction to Thermochemistry PowerPoint Presentation, free Calorimeter How To Find Temperature A practical approximation for the relationship between heat transfer and temperature change is: Read on to learn what calorimetry is and how to solve calorimetry problems with the proper equations. We need to find the difference between the heat lost by the hot water when it droped from 60.0 to 40.0 and the heat gained by the cold water. Calorimetry. Calorimeter How To Find Temperature.
From www.youtube.com
Coffee Cup Calorimeter Calculate Enthalpy Change, Constant Pressure Calorimeter How To Find Temperature To do so, the heat is exchanged with a calibrated object. Read on to learn what calorimetry is and how to solve calorimetry problems with the proper equations. We need to find the difference between the heat lost by the hot water when it droped from 60.0 to 40.0 and the heat gained by the cold water. \ [q =. Calorimeter How To Find Temperature.
From www.youtube.com
How to find calorimeter constant YouTube Calorimeter How To Find Temperature Read on to learn what calorimetry is and how to solve calorimetry problems with the proper equations. We will look at two ways of measuring this heat, either the temperature change of the calorimeter, or the mass of a substance that undergoes a phase change within the. Calorimetry is a science where you try to find. \ [q = mc\delta. Calorimeter How To Find Temperature.
From www.youtube.com
CHEMISTRY 101 Constant volume calorimetry YouTube Calorimeter How To Find Temperature To do so, the heat is exchanged with a calibrated object. Calorimetry is used to measure amounts of heat transferred to or from a substance. A practical approximation for the relationship between heat transfer and temperature change is: We need to find the difference between the heat lost by the hot water when it droped from 60.0 to 40.0 and. Calorimeter How To Find Temperature.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6133898 Calorimeter How To Find Temperature Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of heat released or absorbed and. A practical approximation for the relationship between heat transfer and temperature change is: We need to find the difference between the heat lost by the hot water when it droped from 60.0 to 40.0 and the. Calorimeter How To Find Temperature.
From www.slideserve.com
PPT Unit 13 Thermochemistry PowerPoint Presentation, free download Calorimeter How To Find Temperature To do so, the heat is exchanged with a calibrated object. The temperature change produced by the known reaction is used to determine the heat capacity of the calorimeter. Calorimetry is a science where you try to find. Calorimetry is used to measure amounts of heat transferred to or from a substance. A practical approximation for the relationship between heat. Calorimeter How To Find Temperature.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec Calorimeter How To Find Temperature Calorimetry is a science where you try to find. A practical approximation for the relationship between heat transfer and temperature change is: We need to find the difference between the heat lost by the hot water when it droped from 60.0 to 40.0 and the heat gained by the cold water. To do so, the heat is exchanged with a. Calorimeter How To Find Temperature.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Calorimeter How To Find Temperature Read on to learn what calorimetry is and how to solve calorimetry problems with the proper equations. The temperature change produced by the known reaction is used to determine the heat capacity of the calorimeter. \ [q = mc\delta t,\] where \ (q\) is the symbol for heat transfer (“quantity of heat”), m. We will look at two ways of. Calorimeter How To Find Temperature.