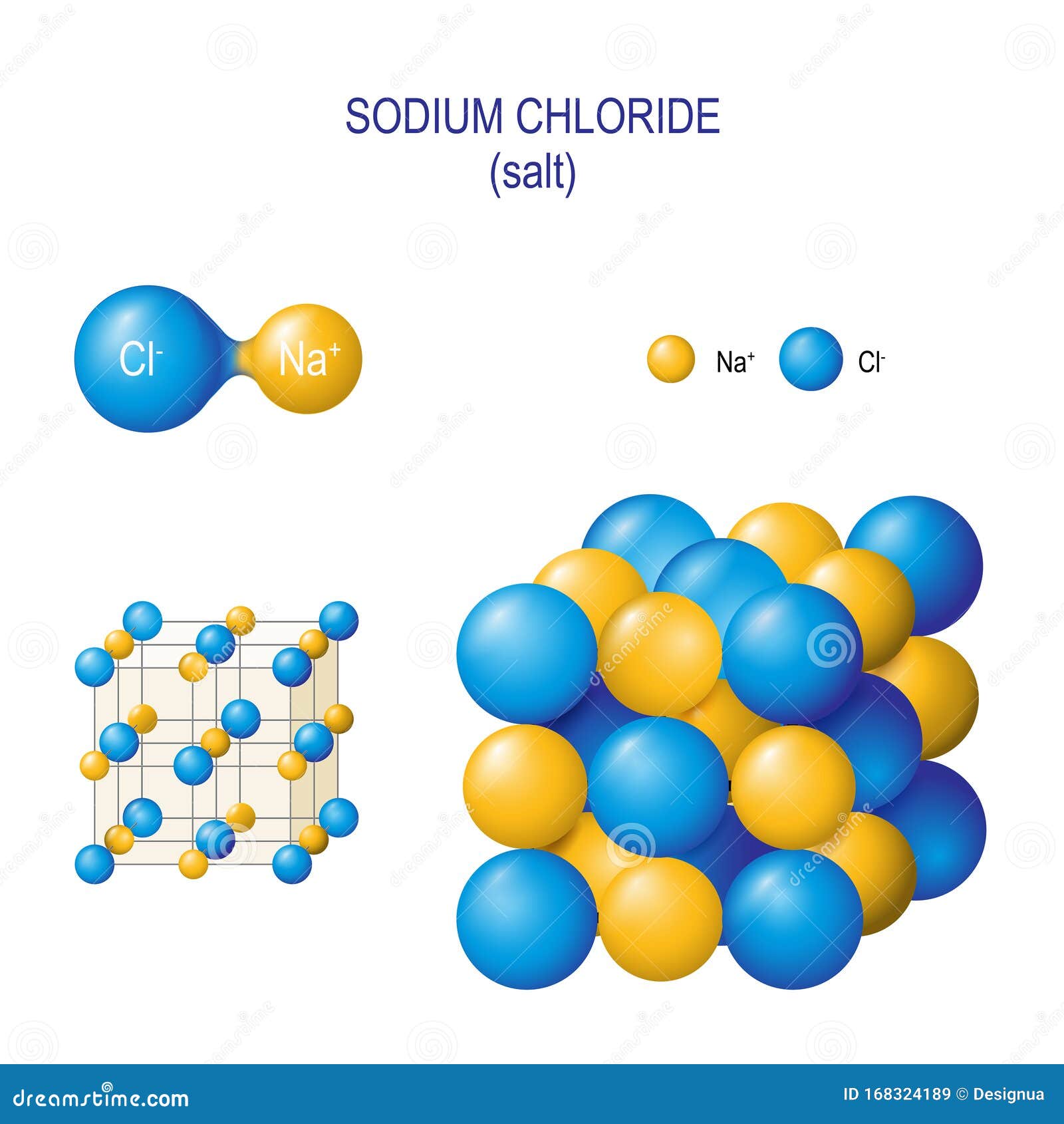
Model Of Sodium Chloride . Nacl 6 and clna 6 octahedra. All octahedral holes in a cubic close packing are occupied by counterions. The intramolecular bonding is ionic, as it involves the. The smaller purple spheres represent sodium cations, \(\ce{na^{+}}\), and the larger green spheres represent chloride anions, \(\ce{cl^{−}}\). Both ions show octahedral coordination (cn = 6). The classic case of ionic bonding, the sodium chloride molecule forms by the ionization of sodium and. in this model, it is a representation of the structure of sodium chloride, an ionic network. The crystal structure of sodium chloride, \(\ce{nacl}\), a typical ionic compound.
from mungfali.com
The classic case of ionic bonding, the sodium chloride molecule forms by the ionization of sodium and. Both ions show octahedral coordination (cn = 6). The smaller purple spheres represent sodium cations, \(\ce{na^{+}}\), and the larger green spheres represent chloride anions, \(\ce{cl^{−}}\). The crystal structure of sodium chloride, \(\ce{nacl}\), a typical ionic compound. Nacl 6 and clna 6 octahedra. The intramolecular bonding is ionic, as it involves the. All octahedral holes in a cubic close packing are occupied by counterions. in this model, it is a representation of the structure of sodium chloride, an ionic network.
Sodium Chloride Molecule Diagram
Model Of Sodium Chloride All octahedral holes in a cubic close packing are occupied by counterions. The crystal structure of sodium chloride, \(\ce{nacl}\), a typical ionic compound. The intramolecular bonding is ionic, as it involves the. All octahedral holes in a cubic close packing are occupied by counterions. The classic case of ionic bonding, the sodium chloride molecule forms by the ionization of sodium and. Both ions show octahedral coordination (cn = 6). Nacl 6 and clna 6 octahedra. The smaller purple spheres represent sodium cations, \(\ce{na^{+}}\), and the larger green spheres represent chloride anions, \(\ce{cl^{−}}\). in this model, it is a representation of the structure of sodium chloride, an ionic network.
From www.alamy.com
molecular model of sodium chloride crystal Stock Photo Alamy Model Of Sodium Chloride Both ions show octahedral coordination (cn = 6). The smaller purple spheres represent sodium cations, \(\ce{na^{+}}\), and the larger green spheres represent chloride anions, \(\ce{cl^{−}}\). The classic case of ionic bonding, the sodium chloride molecule forms by the ionization of sodium and. The crystal structure of sodium chloride, \(\ce{nacl}\), a typical ionic compound. in this model, it is a. Model Of Sodium Chloride.
From sketchfab.com
Sodium Chloride Download Free 3D model by arloopa [fbadfd9] Sketchfab Model Of Sodium Chloride Both ions show octahedral coordination (cn = 6). All octahedral holes in a cubic close packing are occupied by counterions. Nacl 6 and clna 6 octahedra. The classic case of ionic bonding, the sodium chloride molecule forms by the ionization of sodium and. The intramolecular bonding is ionic, as it involves the. in this model, it is a representation. Model Of Sodium Chloride.
From sketchfab.com
Sodium chloride (NaCl) Model A Download Free 3D model by CLauter Model Of Sodium Chloride All octahedral holes in a cubic close packing are occupied by counterions. Nacl 6 and clna 6 octahedra. The intramolecular bonding is ionic, as it involves the. Both ions show octahedral coordination (cn = 6). The crystal structure of sodium chloride, \(\ce{nacl}\), a typical ionic compound. The smaller purple spheres represent sodium cations, \(\ce{na^{+}}\), and the larger green spheres represent. Model Of Sodium Chloride.
From sketchfab.com
Sodium chloride (NaCl) Model G Download Free 3D model by CLauter Model Of Sodium Chloride Both ions show octahedral coordination (cn = 6). All octahedral holes in a cubic close packing are occupied by counterions. The intramolecular bonding is ionic, as it involves the. Nacl 6 and clna 6 octahedra. The smaller purple spheres represent sodium cations, \(\ce{na^{+}}\), and the larger green spheres represent chloride anions, \(\ce{cl^{−}}\). in this model, it is a representation. Model Of Sodium Chloride.
From commons.wikimedia.org
FileSodiumchloride3DvdW.png Wikimedia Commons Model Of Sodium Chloride Nacl 6 and clna 6 octahedra. The classic case of ionic bonding, the sodium chloride molecule forms by the ionization of sodium and. The crystal structure of sodium chloride, \(\ce{nacl}\), a typical ionic compound. The smaller purple spheres represent sodium cations, \(\ce{na^{+}}\), and the larger green spheres represent chloride anions, \(\ce{cl^{−}}\). The intramolecular bonding is ionic, as it involves the.. Model Of Sodium Chloride.
From stock.adobe.com
Sodium chloride, NaCl structure chemistry, Vector illustration. Stock Model Of Sodium Chloride The intramolecular bonding is ionic, as it involves the. Both ions show octahedral coordination (cn = 6). Nacl 6 and clna 6 octahedra. in this model, it is a representation of the structure of sodium chloride, an ionic network. The smaller purple spheres represent sodium cations, \(\ce{na^{+}}\), and the larger green spheres represent chloride anions, \(\ce{cl^{−}}\). The crystal structure. Model Of Sodium Chloride.
From mungfali.com
Sodium Chloride Molecule Diagram Model Of Sodium Chloride in this model, it is a representation of the structure of sodium chloride, an ionic network. Both ions show octahedral coordination (cn = 6). All octahedral holes in a cubic close packing are occupied by counterions. The smaller purple spheres represent sodium cations, \(\ce{na^{+}}\), and the larger green spheres represent chloride anions, \(\ce{cl^{−}}\). The classic case of ionic bonding,. Model Of Sodium Chloride.
From www.shutterstock.com
Crystalline Structure Model Sodium Chloride Molecule Stock Illustration Model Of Sodium Chloride The smaller purple spheres represent sodium cations, \(\ce{na^{+}}\), and the larger green spheres represent chloride anions, \(\ce{cl^{−}}\). Both ions show octahedral coordination (cn = 6). Nacl 6 and clna 6 octahedra. All octahedral holes in a cubic close packing are occupied by counterions. in this model, it is a representation of the structure of sodium chloride, an ionic network.. Model Of Sodium Chloride.
From mungfali.com
Sodium Chloride Molecule Diagram Model Of Sodium Chloride Nacl 6 and clna 6 octahedra. Both ions show octahedral coordination (cn = 6). in this model, it is a representation of the structure of sodium chloride, an ionic network. The intramolecular bonding is ionic, as it involves the. The classic case of ionic bonding, the sodium chloride molecule forms by the ionization of sodium and. The smaller purple. Model Of Sodium Chloride.
From www.alamy.com
Molecular Model of Sodium Chloride (NaCl) Molecule. Vector Illustration Model Of Sodium Chloride The crystal structure of sodium chloride, \(\ce{nacl}\), a typical ionic compound. Both ions show octahedral coordination (cn = 6). The smaller purple spheres represent sodium cations, \(\ce{na^{+}}\), and the larger green spheres represent chloride anions, \(\ce{cl^{−}}\). The intramolecular bonding is ionic, as it involves the. in this model, it is a representation of the structure of sodium chloride, an. Model Of Sodium Chloride.
From mungfali.com
Sodium Chloride Molecule Diagram Model Of Sodium Chloride Both ions show octahedral coordination (cn = 6). Nacl 6 and clna 6 octahedra. The classic case of ionic bonding, the sodium chloride molecule forms by the ionization of sodium and. The crystal structure of sodium chloride, \(\ce{nacl}\), a typical ionic compound. The smaller purple spheres represent sodium cations, \(\ce{na^{+}}\), and the larger green spheres represent chloride anions, \(\ce{cl^{−}}\). All. Model Of Sodium Chloride.
From www.gettyimages.co.uk
Sodium Chloride Nacl Molecular Structure HighRes Vector Graphic Model Of Sodium Chloride Nacl 6 and clna 6 octahedra. All octahedral holes in a cubic close packing are occupied by counterions. Both ions show octahedral coordination (cn = 6). The crystal structure of sodium chloride, \(\ce{nacl}\), a typical ionic compound. The classic case of ionic bonding, the sodium chloride molecule forms by the ionization of sodium and. in this model, it is. Model Of Sodium Chloride.
From www.alamy.com
Molecular Model of Sodium Chloride (NaCl) Molecule. Vector Illustration Model Of Sodium Chloride in this model, it is a representation of the structure of sodium chloride, an ionic network. All octahedral holes in a cubic close packing are occupied by counterions. Nacl 6 and clna 6 octahedra. The classic case of ionic bonding, the sodium chloride molecule forms by the ionization of sodium and. The smaller purple spheres represent sodium cations, \(\ce{na^{+}}\),. Model Of Sodium Chloride.
From www.turbosquid.com
3ds molecular sodium chloride Model Of Sodium Chloride Both ions show octahedral coordination (cn = 6). Nacl 6 and clna 6 octahedra. The crystal structure of sodium chloride, \(\ce{nacl}\), a typical ionic compound. The classic case of ionic bonding, the sodium chloride molecule forms by the ionization of sodium and. The intramolecular bonding is ionic, as it involves the. All octahedral holes in a cubic close packing are. Model Of Sodium Chloride.
From mungfali.com
Molecular Structure Of Sodium Chloride Model Of Sodium Chloride The classic case of ionic bonding, the sodium chloride molecule forms by the ionization of sodium and. The intramolecular bonding is ionic, as it involves the. in this model, it is a representation of the structure of sodium chloride, an ionic network. The smaller purple spheres represent sodium cations, \(\ce{na^{+}}\), and the larger green spheres represent chloride anions, \(\ce{cl^{−}}\).. Model Of Sodium Chloride.
From parcoscientific.com
Sodium Chloride Molecular Model Molecular Models Chemistry Model Of Sodium Chloride Nacl 6 and clna 6 octahedra. Both ions show octahedral coordination (cn = 6). The classic case of ionic bonding, the sodium chloride molecule forms by the ionization of sodium and. The smaller purple spheres represent sodium cations, \(\ce{na^{+}}\), and the larger green spheres represent chloride anions, \(\ce{cl^{−}}\). The crystal structure of sodium chloride, \(\ce{nacl}\), a typical ionic compound. All. Model Of Sodium Chloride.
From www.alamy.com
Crystal structure of sodium chloride, illustration Stock Photo Alamy Model Of Sodium Chloride Nacl 6 and clna 6 octahedra. The intramolecular bonding is ionic, as it involves the. Both ions show octahedral coordination (cn = 6). All octahedral holes in a cubic close packing are occupied by counterions. The crystal structure of sodium chloride, \(\ce{nacl}\), a typical ionic compound. The smaller purple spheres represent sodium cations, \(\ce{na^{+}}\), and the larger green spheres represent. Model Of Sodium Chloride.
From www.sciencephoto.com
Sodium chloride, molecular model Stock Image C028/8132 Science Model Of Sodium Chloride All octahedral holes in a cubic close packing are occupied by counterions. The crystal structure of sodium chloride, \(\ce{nacl}\), a typical ionic compound. The intramolecular bonding is ionic, as it involves the. in this model, it is a representation of the structure of sodium chloride, an ionic network. The smaller purple spheres represent sodium cations, \(\ce{na^{+}}\), and the larger. Model Of Sodium Chloride.
From www.alamy.com
Sodium chloride (NaCl, table salt), crystal structure. Atoms are Model Of Sodium Chloride All octahedral holes in a cubic close packing are occupied by counterions. The smaller purple spheres represent sodium cations, \(\ce{na^{+}}\), and the larger green spheres represent chloride anions, \(\ce{cl^{−}}\). in this model, it is a representation of the structure of sodium chloride, an ionic network. The intramolecular bonding is ionic, as it involves the. The crystal structure of sodium. Model Of Sodium Chloride.
From www.westlab.com
Sodium Chloride Crystal Model Westlab Model Of Sodium Chloride in this model, it is a representation of the structure of sodium chloride, an ionic network. The intramolecular bonding is ionic, as it involves the. The classic case of ionic bonding, the sodium chloride molecule forms by the ionization of sodium and. The smaller purple spheres represent sodium cations, \(\ce{na^{+}}\), and the larger green spheres represent chloride anions, \(\ce{cl^{−}}\).. Model Of Sodium Chloride.
From sketchfab.com
Crystalline Structure Sodium Chloride Download Free 3D model by Model Of Sodium Chloride The intramolecular bonding is ionic, as it involves the. The smaller purple spheres represent sodium cations, \(\ce{na^{+}}\), and the larger green spheres represent chloride anions, \(\ce{cl^{−}}\). The classic case of ionic bonding, the sodium chloride molecule forms by the ionization of sodium and. in this model, it is a representation of the structure of sodium chloride, an ionic network.. Model Of Sodium Chloride.
From www.alamy.com
Sodium Chloride NaCl Salt Sodium and Chloride ions forming three Model Of Sodium Chloride The smaller purple spheres represent sodium cations, \(\ce{na^{+}}\), and the larger green spheres represent chloride anions, \(\ce{cl^{−}}\). The classic case of ionic bonding, the sodium chloride molecule forms by the ionization of sodium and. in this model, it is a representation of the structure of sodium chloride, an ionic network. Both ions show octahedral coordination (cn = 6). The. Model Of Sodium Chloride.
From www.alamy.com
Molecular Model of Sodium Chloride (NaCl) Molecule. Vector Illustration Model Of Sodium Chloride The intramolecular bonding is ionic, as it involves the. The smaller purple spheres represent sodium cations, \(\ce{na^{+}}\), and the larger green spheres represent chloride anions, \(\ce{cl^{−}}\). in this model, it is a representation of the structure of sodium chloride, an ionic network. Both ions show octahedral coordination (cn = 6). The classic case of ionic bonding, the sodium chloride. Model Of Sodium Chloride.
From en.wikidoc.org
Sodium chloride wikidoc Model Of Sodium Chloride All octahedral holes in a cubic close packing are occupied by counterions. The smaller purple spheres represent sodium cations, \(\ce{na^{+}}\), and the larger green spheres represent chloride anions, \(\ce{cl^{−}}\). The crystal structure of sodium chloride, \(\ce{nacl}\), a typical ionic compound. The classic case of ionic bonding, the sodium chloride molecule forms by the ionization of sodium and. Nacl 6 and. Model Of Sodium Chloride.
From sketchfab.com
Sodium Chloride NaCl 3D model by megann_ [b6ddb73] Sketchfab Model Of Sodium Chloride in this model, it is a representation of the structure of sodium chloride, an ionic network. The intramolecular bonding is ionic, as it involves the. The smaller purple spheres represent sodium cations, \(\ce{na^{+}}\), and the larger green spheres represent chloride anions, \(\ce{cl^{−}}\). Both ions show octahedral coordination (cn = 6). The crystal structure of sodium chloride, \(\ce{nacl}\), a typical. Model Of Sodium Chloride.
From mavink.com
Sodium Molecule Diagram Model Of Sodium Chloride in this model, it is a representation of the structure of sodium chloride, an ionic network. The classic case of ionic bonding, the sodium chloride molecule forms by the ionization of sodium and. The crystal structure of sodium chloride, \(\ce{nacl}\), a typical ionic compound. All octahedral holes in a cubic close packing are occupied by counterions. The intramolecular bonding. Model Of Sodium Chloride.
From www.alamy.com
Molecular Model of Sodium Chloride (NaCl) Molecule. Vector Illustration Model Of Sodium Chloride All octahedral holes in a cubic close packing are occupied by counterions. The classic case of ionic bonding, the sodium chloride molecule forms by the ionization of sodium and. The crystal structure of sodium chloride, \(\ce{nacl}\), a typical ionic compound. in this model, it is a representation of the structure of sodium chloride, an ionic network. Nacl 6 and. Model Of Sodium Chloride.
From www.shutterstock.com
Illustration Sodium Chloride Crystal Structure Stock Vector 50841040 Model Of Sodium Chloride The classic case of ionic bonding, the sodium chloride molecule forms by the ionization of sodium and. Nacl 6 and clna 6 octahedra. Both ions show octahedral coordination (cn = 6). All octahedral holes in a cubic close packing are occupied by counterions. The smaller purple spheres represent sodium cations, \(\ce{na^{+}}\), and the larger green spheres represent chloride anions, \(\ce{cl^{−}}\).. Model Of Sodium Chloride.
From commons.wikimedia.org
FileSodiumchlorideunitcell3Dionic.png Wikimedia Commons Model Of Sodium Chloride The smaller purple spheres represent sodium cations, \(\ce{na^{+}}\), and the larger green spheres represent chloride anions, \(\ce{cl^{−}}\). The crystal structure of sodium chloride, \(\ce{nacl}\), a typical ionic compound. The classic case of ionic bonding, the sodium chloride molecule forms by the ionization of sodium and. Both ions show octahedral coordination (cn = 6). Nacl 6 and clna 6 octahedra. . Model Of Sodium Chloride.
From www.3bscientific.com
Sodium Chloride Model Molecular Models Model Of Sodium Chloride Both ions show octahedral coordination (cn = 6). The smaller purple spheres represent sodium cations, \(\ce{na^{+}}\), and the larger green spheres represent chloride anions, \(\ce{cl^{−}}\). All octahedral holes in a cubic close packing are occupied by counterions. The classic case of ionic bonding, the sodium chloride molecule forms by the ionization of sodium and. The crystal structure of sodium chloride,. Model Of Sodium Chloride.
From www.sciencephoto.com
Model of sodium chloride crystal lattice Stock Image A504/0012 Model Of Sodium Chloride Nacl 6 and clna 6 octahedra. The classic case of ionic bonding, the sodium chloride molecule forms by the ionization of sodium and. Both ions show octahedral coordination (cn = 6). All octahedral holes in a cubic close packing are occupied by counterions. in this model, it is a representation of the structure of sodium chloride, an ionic network.. Model Of Sodium Chloride.
From mungfali.com
Sodium Chloride Molecule Diagram Model Of Sodium Chloride All octahedral holes in a cubic close packing are occupied by counterions. The intramolecular bonding is ionic, as it involves the. The smaller purple spheres represent sodium cations, \(\ce{na^{+}}\), and the larger green spheres represent chloride anions, \(\ce{cl^{−}}\). Both ions show octahedral coordination (cn = 6). The classic case of ionic bonding, the sodium chloride molecule forms by the ionization. Model Of Sodium Chloride.
From mungfali.com
Sodium Chloride Molecule Diagram Model Of Sodium Chloride in this model, it is a representation of the structure of sodium chloride, an ionic network. Both ions show octahedral coordination (cn = 6). The smaller purple spheres represent sodium cations, \(\ce{na^{+}}\), and the larger green spheres represent chloride anions, \(\ce{cl^{−}}\). The classic case of ionic bonding, the sodium chloride molecule forms by the ionization of sodium and. Nacl. Model Of Sodium Chloride.
From mungfali.com
Sodium Chloride Molecule Diagram Model Of Sodium Chloride Nacl 6 and clna 6 octahedra. The intramolecular bonding is ionic, as it involves the. The smaller purple spheres represent sodium cations, \(\ce{na^{+}}\), and the larger green spheres represent chloride anions, \(\ce{cl^{−}}\). The crystal structure of sodium chloride, \(\ce{nacl}\), a typical ionic compound. Both ions show octahedral coordination (cn = 6). in this model, it is a representation of. Model Of Sodium Chloride.
From www.dreamstime.com
Sodium Chloride Molecule Model Structure Stock Vector Illustration of Model Of Sodium Chloride Nacl 6 and clna 6 octahedra. The intramolecular bonding is ionic, as it involves the. Both ions show octahedral coordination (cn = 6). The crystal structure of sodium chloride, \(\ce{nacl}\), a typical ionic compound. The smaller purple spheres represent sodium cations, \(\ce{na^{+}}\), and the larger green spheres represent chloride anions, \(\ce{cl^{−}}\). The classic case of ionic bonding, the sodium chloride. Model Of Sodium Chloride.