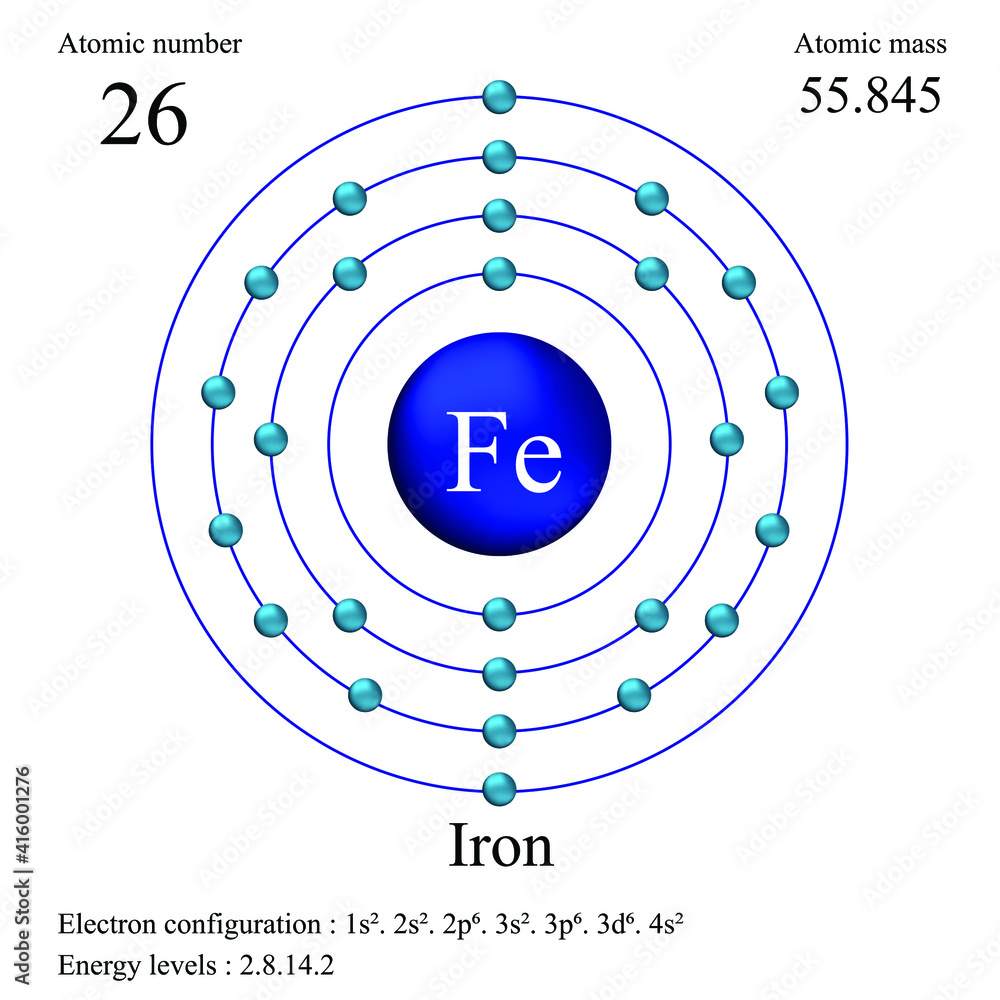
Atomic Number For Iron Neutrons . These numbers vary when iron is in any of its ionic or isotopic. All atomic nuclei of the chemical element iron are summarized under iron isotopes; Use a periodic table to calculate the mass number of a hydrogen atom that contains 2 neutrons. Neutron number and mass number of iron. These consist of an atomic nucleus with 26 protons and in the. The mass number of an atom is calculated by adding together the number of protons. Mass numbers of typical isotopes of iron are 56; The total number of neutrons in.
from makeup.vidalondon.net
These numbers vary when iron is in any of its ionic or isotopic. The mass number of an atom is calculated by adding together the number of protons. These consist of an atomic nucleus with 26 protons and in the. Neutron number and mass number of iron. All atomic nuclei of the chemical element iron are summarized under iron isotopes; Use a periodic table to calculate the mass number of a hydrogen atom that contains 2 neutrons. The total number of neutrons in. Mass numbers of typical isotopes of iron are 56;
What Is The Atomic Makeup Of Iron Makeup Vidalondon
Atomic Number For Iron Neutrons The total number of neutrons in. All atomic nuclei of the chemical element iron are summarized under iron isotopes; These numbers vary when iron is in any of its ionic or isotopic. The total number of neutrons in. Neutron number and mass number of iron. Use a periodic table to calculate the mass number of a hydrogen atom that contains 2 neutrons. Mass numbers of typical isotopes of iron are 56; These consist of an atomic nucleus with 26 protons and in the. The mass number of an atom is calculated by adding together the number of protons.
From finwise.edu.vn
Collection 95+ Pictures Number Of Protons Neutrons And Electrons In Atomic Number For Iron Neutrons Neutron number and mass number of iron. Mass numbers of typical isotopes of iron are 56; The total number of neutrons in. The mass number of an atom is calculated by adding together the number of protons. All atomic nuclei of the chemical element iron are summarized under iron isotopes; These consist of an atomic nucleus with 26 protons and. Atomic Number For Iron Neutrons.
From www.numerade.com
SOLVED 'Using the periodic table entry of iron below, match the Atomic Number For Iron Neutrons Mass numbers of typical isotopes of iron are 56; The mass number of an atom is calculated by adding together the number of protons. All atomic nuclei of the chemical element iron are summarized under iron isotopes; These consist of an atomic nucleus with 26 protons and in the. Neutron number and mass number of iron. Use a periodic table. Atomic Number For Iron Neutrons.
From www.slideserve.com
PPT How many protons , electrons, and neutrons are in an atom Atomic Number For Iron Neutrons The mass number of an atom is calculated by adding together the number of protons. Mass numbers of typical isotopes of iron are 56; These numbers vary when iron is in any of its ionic or isotopic. These consist of an atomic nucleus with 26 protons and in the. Neutron number and mass number of iron. All atomic nuclei of. Atomic Number For Iron Neutrons.
From www.slideserve.com
PPT 1. What does the atomic number of an element represent Atomic Number For Iron Neutrons Use a periodic table to calculate the mass number of a hydrogen atom that contains 2 neutrons. The mass number of an atom is calculated by adding together the number of protons. These consist of an atomic nucleus with 26 protons and in the. The total number of neutrons in. Mass numbers of typical isotopes of iron are 56; These. Atomic Number For Iron Neutrons.
From www.baamboozle.com
Atomic Structure and Elements Baamboozle Baamboozle The Most Fun Atomic Number For Iron Neutrons Use a periodic table to calculate the mass number of a hydrogen atom that contains 2 neutrons. Mass numbers of typical isotopes of iron are 56; All atomic nuclei of the chemical element iron are summarized under iron isotopes; The mass number of an atom is calculated by adding together the number of protons. The total number of neutrons in.. Atomic Number For Iron Neutrons.
From slidetodoc.com
How to find the number of protons neutrons Atomic Number For Iron Neutrons Use a periodic table to calculate the mass number of a hydrogen atom that contains 2 neutrons. Neutron number and mass number of iron. These numbers vary when iron is in any of its ionic or isotopic. These consist of an atomic nucleus with 26 protons and in the. Mass numbers of typical isotopes of iron are 56; All atomic. Atomic Number For Iron Neutrons.
From reviewhomedecor.co
Iron Periodic Table Number Of Neutrons Review Home Decor Atomic Number For Iron Neutrons These consist of an atomic nucleus with 26 protons and in the. The mass number of an atom is calculated by adding together the number of protons. The total number of neutrons in. Use a periodic table to calculate the mass number of a hydrogen atom that contains 2 neutrons. These numbers vary when iron is in any of its. Atomic Number For Iron Neutrons.
From slidetodoc.com
How to find the number of protons neutrons Atomic Number For Iron Neutrons The total number of neutrons in. These consist of an atomic nucleus with 26 protons and in the. Neutron number and mass number of iron. Use a periodic table to calculate the mass number of a hydrogen atom that contains 2 neutrons. Mass numbers of typical isotopes of iron are 56; All atomic nuclei of the chemical element iron are. Atomic Number For Iron Neutrons.
From makeup.vidalondon.net
What Is The Atomic Makeup Of Iron Makeup Vidalondon Atomic Number For Iron Neutrons Neutron number and mass number of iron. Use a periodic table to calculate the mass number of a hydrogen atom that contains 2 neutrons. Mass numbers of typical isotopes of iron are 56; The mass number of an atom is calculated by adding together the number of protons. All atomic nuclei of the chemical element iron are summarized under iron. Atomic Number For Iron Neutrons.
From valenceelectrons.com
Electron Configuration for Iron (Fe and Fe2+, Fe3+ ions) Atomic Number For Iron Neutrons All atomic nuclei of the chemical element iron are summarized under iron isotopes; The mass number of an atom is calculated by adding together the number of protons. These numbers vary when iron is in any of its ionic or isotopic. These consist of an atomic nucleus with 26 protons and in the. Mass numbers of typical isotopes of iron. Atomic Number For Iron Neutrons.
From www.britannica.com
Lead Definition, Uses, Properties, & Facts Britannica Atomic Number For Iron Neutrons The total number of neutrons in. All atomic nuclei of the chemical element iron are summarized under iron isotopes; Neutron number and mass number of iron. Mass numbers of typical isotopes of iron are 56; Use a periodic table to calculate the mass number of a hydrogen atom that contains 2 neutrons. These numbers vary when iron is in any. Atomic Number For Iron Neutrons.
From chemistry291.blogspot.com
How Many Neutrons Does Iron(Fe) Have?Number of Neutrons in Iron(Fe) Atomic Number For Iron Neutrons Mass numbers of typical isotopes of iron are 56; The total number of neutrons in. The mass number of an atom is calculated by adding together the number of protons. These consist of an atomic nucleus with 26 protons and in the. Use a periodic table to calculate the mass number of a hydrogen atom that contains 2 neutrons. All. Atomic Number For Iron Neutrons.
From material-properties.org
Hierro Tabla periódica y propiedades atómicas Atomic Number For Iron Neutrons Use a periodic table to calculate the mass number of a hydrogen atom that contains 2 neutrons. These numbers vary when iron is in any of its ionic or isotopic. These consist of an atomic nucleus with 26 protons and in the. The mass number of an atom is calculated by adding together the number of protons. Mass numbers of. Atomic Number For Iron Neutrons.
From socratic.org
Why don't any of the isotopes of natural iron have the atomic mass of Atomic Number For Iron Neutrons The total number of neutrons in. Mass numbers of typical isotopes of iron are 56; All atomic nuclei of the chemical element iron are summarized under iron isotopes; Neutron number and mass number of iron. These consist of an atomic nucleus with 26 protons and in the. These numbers vary when iron is in any of its ionic or isotopic.. Atomic Number For Iron Neutrons.
From www.sciencephoto.com
Iron, atomic structure Stock Image C018/3707 Science Photo Library Atomic Number For Iron Neutrons The mass number of an atom is calculated by adding together the number of protons. Neutron number and mass number of iron. These numbers vary when iron is in any of its ionic or isotopic. These consist of an atomic nucleus with 26 protons and in the. The total number of neutrons in. Use a periodic table to calculate the. Atomic Number For Iron Neutrons.
From www.alamy.com
Iron chemical element, Sign with atomic number and atomic weight Atomic Number For Iron Neutrons Mass numbers of typical isotopes of iron are 56; Neutron number and mass number of iron. These consist of an atomic nucleus with 26 protons and in the. Use a periodic table to calculate the mass number of a hydrogen atom that contains 2 neutrons. These numbers vary when iron is in any of its ionic or isotopic. The mass. Atomic Number For Iron Neutrons.
From reviewhomedecor.co
Iron Periodic Table Number Of Neutrons Review Home Decor Atomic Number For Iron Neutrons These numbers vary when iron is in any of its ionic or isotopic. Use a periodic table to calculate the mass number of a hydrogen atom that contains 2 neutrons. The mass number of an atom is calculated by adding together the number of protons. All atomic nuclei of the chemical element iron are summarized under iron isotopes; The total. Atomic Number For Iron Neutrons.
From reviewhomedecor.co
Iron Periodic Table Number Of Neutrons Review Home Decor Atomic Number For Iron Neutrons Neutron number and mass number of iron. These consist of an atomic nucleus with 26 protons and in the. The mass number of an atom is calculated by adding together the number of protons. These numbers vary when iron is in any of its ionic or isotopic. Use a periodic table to calculate the mass number of a hydrogen atom. Atomic Number For Iron Neutrons.
From reviewhomedecor.co
Iron Periodic Table Number Of Neutrons Review Home Decor Atomic Number For Iron Neutrons Neutron number and mass number of iron. All atomic nuclei of the chemical element iron are summarized under iron isotopes; Mass numbers of typical isotopes of iron are 56; The mass number of an atom is calculated by adding together the number of protons. These consist of an atomic nucleus with 26 protons and in the. The total number of. Atomic Number For Iron Neutrons.
From www.wikihow.com
How to Find the Number of Neutrons in an Atom 11 Steps Atomic Number For Iron Neutrons The total number of neutrons in. These numbers vary when iron is in any of its ionic or isotopic. Mass numbers of typical isotopes of iron are 56; All atomic nuclei of the chemical element iron are summarized under iron isotopes; Neutron number and mass number of iron. The mass number of an atom is calculated by adding together the. Atomic Number For Iron Neutrons.
From thptlaihoa.edu.vn
How to Find the Number of Neutrons in an Atom Atomic Number For Iron Neutrons These consist of an atomic nucleus with 26 protons and in the. Mass numbers of typical isotopes of iron are 56; The mass number of an atom is calculated by adding together the number of protons. Neutron number and mass number of iron. The total number of neutrons in. Use a periodic table to calculate the mass number of a. Atomic Number For Iron Neutrons.
From reviewhomedecor.co
Silver Periodic Table Number Of Neutrons Review Home Decor Atomic Number For Iron Neutrons Use a periodic table to calculate the mass number of a hydrogen atom that contains 2 neutrons. The mass number of an atom is calculated by adding together the number of protons. These consist of an atomic nucleus with 26 protons and in the. Mass numbers of typical isotopes of iron are 56; Neutron number and mass number of iron.. Atomic Number For Iron Neutrons.
From reviewhomedecor.co
Iron Periodic Table Protons Neutrons Electrons Review Home Decor Atomic Number For Iron Neutrons Mass numbers of typical isotopes of iron are 56; Use a periodic table to calculate the mass number of a hydrogen atom that contains 2 neutrons. All atomic nuclei of the chemical element iron are summarized under iron isotopes; The mass number of an atom is calculated by adding together the number of protons. The total number of neutrons in.. Atomic Number For Iron Neutrons.
From reviewhomedecor.co
Iron Periodic Table Number Of Neutrons Review Home Decor Atomic Number For Iron Neutrons The total number of neutrons in. These consist of an atomic nucleus with 26 protons and in the. Use a periodic table to calculate the mass number of a hydrogen atom that contains 2 neutrons. These numbers vary when iron is in any of its ionic or isotopic. All atomic nuclei of the chemical element iron are summarized under iron. Atomic Number For Iron Neutrons.
From awesomehome.co
Periodic Table Iron Number Of Neutrons Awesome Home Atomic Number For Iron Neutrons The total number of neutrons in. These numbers vary when iron is in any of its ionic or isotopic. Mass numbers of typical isotopes of iron are 56; These consist of an atomic nucleus with 26 protons and in the. Use a periodic table to calculate the mass number of a hydrogen atom that contains 2 neutrons. Neutron number and. Atomic Number For Iron Neutrons.
From marcokruwguerrero.blogspot.com
Protons Neutrons and Electrons MarcokruwGuerrero Atomic Number For Iron Neutrons Mass numbers of typical isotopes of iron are 56; The total number of neutrons in. These numbers vary when iron is in any of its ionic or isotopic. All atomic nuclei of the chemical element iron are summarized under iron isotopes; These consist of an atomic nucleus with 26 protons and in the. The mass number of an atom is. Atomic Number For Iron Neutrons.
From reviewhomedecor.co
Iron Periodic Table Number Of Neutrons Review Home Decor Atomic Number For Iron Neutrons The mass number of an atom is calculated by adding together the number of protons. These consist of an atomic nucleus with 26 protons and in the. Neutron number and mass number of iron. Use a periodic table to calculate the mass number of a hydrogen atom that contains 2 neutrons. All atomic nuclei of the chemical element iron are. Atomic Number For Iron Neutrons.
From www.numerade.com
SOLVED An iron atom has an atomic mass of 56. its atomic number is 26 Atomic Number For Iron Neutrons The total number of neutrons in. Mass numbers of typical isotopes of iron are 56; These numbers vary when iron is in any of its ionic or isotopic. Use a periodic table to calculate the mass number of a hydrogen atom that contains 2 neutrons. These consist of an atomic nucleus with 26 protons and in the. Neutron number and. Atomic Number For Iron Neutrons.
From valenceelectrons.com
How Many Protons, Neutrons and Electrons Does Iron Have? Atomic Number For Iron Neutrons All atomic nuclei of the chemical element iron are summarized under iron isotopes; Mass numbers of typical isotopes of iron are 56; Use a periodic table to calculate the mass number of a hydrogen atom that contains 2 neutrons. The total number of neutrons in. The mass number of an atom is calculated by adding together the number of protons.. Atomic Number For Iron Neutrons.
From sciencenotes.org
Iron Facts Atomic Number 26 or Fe Atomic Number For Iron Neutrons All atomic nuclei of the chemical element iron are summarized under iron isotopes; Neutron number and mass number of iron. The mass number of an atom is calculated by adding together the number of protons. Mass numbers of typical isotopes of iron are 56; These consist of an atomic nucleus with 26 protons and in the. These numbers vary when. Atomic Number For Iron Neutrons.
From anelementaday.wordpress.com
Day 3 Iron An Element A Day Atomic Number For Iron Neutrons The mass number of an atom is calculated by adding together the number of protons. Neutron number and mass number of iron. These numbers vary when iron is in any of its ionic or isotopic. Mass numbers of typical isotopes of iron are 56; These consist of an atomic nucleus with 26 protons and in the. All atomic nuclei of. Atomic Number For Iron Neutrons.
From brokeasshome.com
Periodic Table Sulfur Protons Neutrons Electrons Atomic Number For Iron Neutrons Use a periodic table to calculate the mass number of a hydrogen atom that contains 2 neutrons. The total number of neutrons in. Neutron number and mass number of iron. The mass number of an atom is calculated by adding together the number of protons. Mass numbers of typical isotopes of iron are 56; These consist of an atomic nucleus. Atomic Number For Iron Neutrons.
From www.expii.com
Neutrons — Structure & Properties Expii Atomic Number For Iron Neutrons These consist of an atomic nucleus with 26 protons and in the. Neutron number and mass number of iron. These numbers vary when iron is in any of its ionic or isotopic. Use a periodic table to calculate the mass number of a hydrogen atom that contains 2 neutrons. Mass numbers of typical isotopes of iron are 56; The mass. Atomic Number For Iron Neutrons.
From animalia-life.club
Isotope Symbol Examples Atomic Number For Iron Neutrons Neutron number and mass number of iron. These numbers vary when iron is in any of its ionic or isotopic. These consist of an atomic nucleus with 26 protons and in the. Mass numbers of typical isotopes of iron are 56; The mass number of an atom is calculated by adding together the number of protons. Use a periodic table. Atomic Number For Iron Neutrons.
From www.bigstockphoto.com
3d Render Atom Structure Iron Image & Photo Bigstock Atomic Number For Iron Neutrons The mass number of an atom is calculated by adding together the number of protons. Neutron number and mass number of iron. The total number of neutrons in. These consist of an atomic nucleus with 26 protons and in the. These numbers vary when iron is in any of its ionic or isotopic. All atomic nuclei of the chemical element. Atomic Number For Iron Neutrons.