Lithium Is Gas Or Not . Lithium has a melting point of 180.54 c, a boiling point of 1342 c, a specific gravity of 0.534 (20 c), and a. Under the proper conditions, the element also combines with sulfur,. Lithium does not react with oxygen at room temperature, but above 100°c does so to form lithium oxide (li 2 0). Lithium is atomic number 3 on the periodic table with element symbol li. Pure lithium will form lithium hydroxide due to moisture in the air, as well as lithium nitride (\(li_3n\)) from \(n_2\) gas, and lithium carbonate \((li_2co_3\)) from carbon dioxide. I understand that it exhibits metallic bonding, but why wouldn't it form a diatomic covalent bond with itself like. Most lithium is currently produced in. Spodumene, petalite, lepidolite, and amblygonite are the more important minerals containing lithium. Why isn't lithium a gas? Here are a collection of lithium facts, including its properties, uses, and sources.
from synergyfiles.com
Spodumene, petalite, lepidolite, and amblygonite are the more important minerals containing lithium. Why isn't lithium a gas? Lithium has a melting point of 180.54 c, a boiling point of 1342 c, a specific gravity of 0.534 (20 c), and a. Here are a collection of lithium facts, including its properties, uses, and sources. Lithium does not react with oxygen at room temperature, but above 100°c does so to form lithium oxide (li 2 0). Most lithium is currently produced in. Under the proper conditions, the element also combines with sulfur,. Lithium is atomic number 3 on the periodic table with element symbol li. I understand that it exhibits metallic bonding, but why wouldn't it form a diatomic covalent bond with itself like. Pure lithium will form lithium hydroxide due to moisture in the air, as well as lithium nitride (\(li_3n\)) from \(n_2\) gas, and lithium carbonate \((li_2co_3\)) from carbon dioxide.
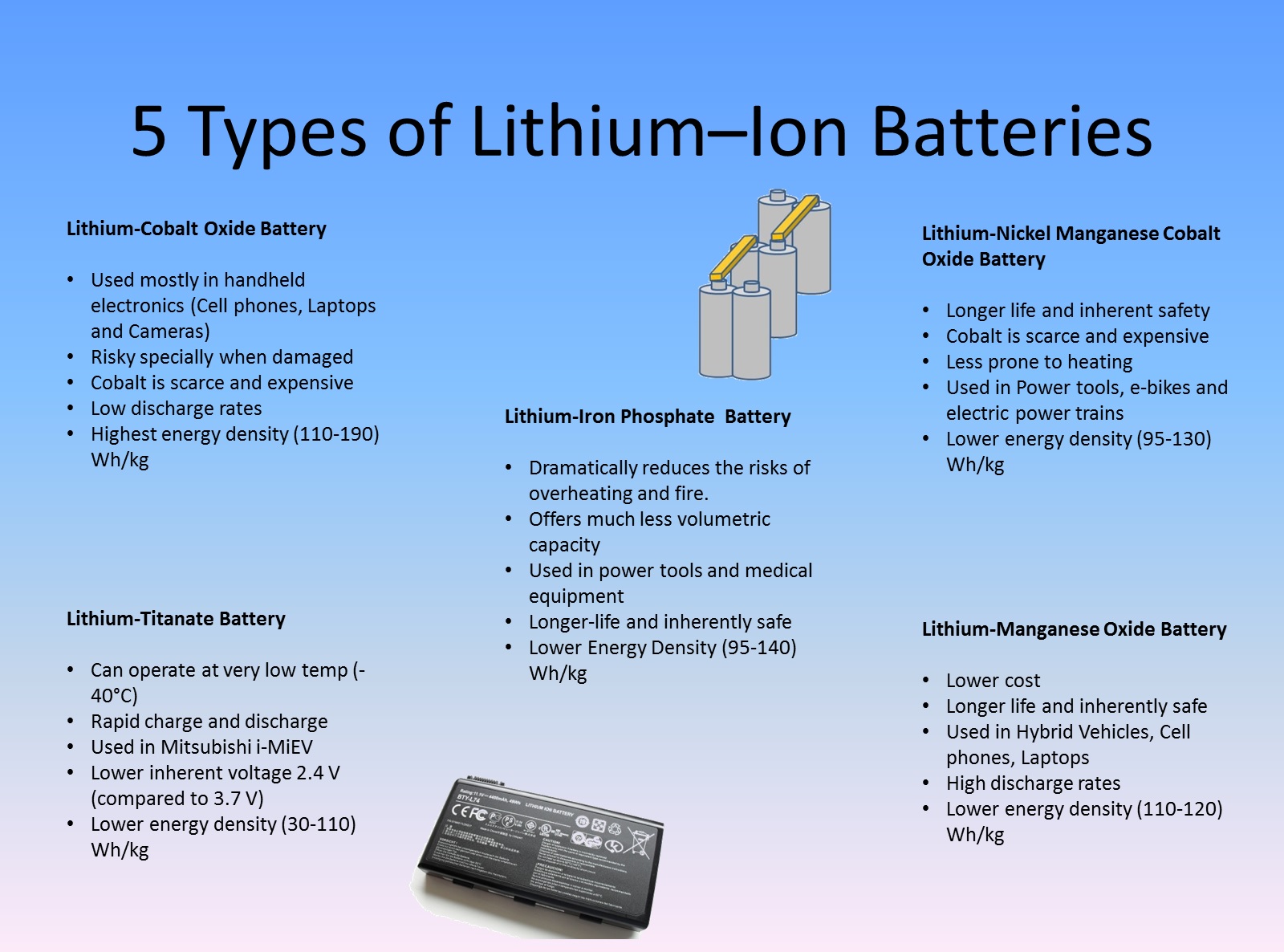
5 Types of Lithium Ion Batteries
Lithium Is Gas Or Not Here are a collection of lithium facts, including its properties, uses, and sources. I understand that it exhibits metallic bonding, but why wouldn't it form a diatomic covalent bond with itself like. Why isn't lithium a gas? Spodumene, petalite, lepidolite, and amblygonite are the more important minerals containing lithium. Under the proper conditions, the element also combines with sulfur,. Lithium does not react with oxygen at room temperature, but above 100°c does so to form lithium oxide (li 2 0). Lithium has a melting point of 180.54 c, a boiling point of 1342 c, a specific gravity of 0.534 (20 c), and a. Here are a collection of lithium facts, including its properties, uses, and sources. Pure lithium will form lithium hydroxide due to moisture in the air, as well as lithium nitride (\(li_3n\)) from \(n_2\) gas, and lithium carbonate \((li_2co_3\)) from carbon dioxide. Lithium is atomic number 3 on the periodic table with element symbol li. Most lithium is currently produced in.
From material-properties.org
Lithium Periodic Table and Atomic Properties Lithium Is Gas Or Not I understand that it exhibits metallic bonding, but why wouldn't it form a diatomic covalent bond with itself like. Under the proper conditions, the element also combines with sulfur,. Pure lithium will form lithium hydroxide due to moisture in the air, as well as lithium nitride (\(li_3n\)) from \(n_2\) gas, and lithium carbonate \((li_2co_3\)) from carbon dioxide. Spodumene, petalite, lepidolite,. Lithium Is Gas Or Not.
From www.solvedlib.com
The reaction of lithium metal and water to form lithi… SolvedLib Lithium Is Gas Or Not I understand that it exhibits metallic bonding, but why wouldn't it form a diatomic covalent bond with itself like. Here are a collection of lithium facts, including its properties, uses, and sources. Under the proper conditions, the element also combines with sulfur,. Lithium is atomic number 3 on the periodic table with element symbol li. Lithium does not react with. Lithium Is Gas Or Not.
From www.sciencephoto.com
Lithium, atomic structure Stock Image C018/3684 Science Photo Library Lithium Is Gas Or Not Lithium does not react with oxygen at room temperature, but above 100°c does so to form lithium oxide (li 2 0). Here are a collection of lithium facts, including its properties, uses, and sources. Lithium has a melting point of 180.54 c, a boiling point of 1342 c, a specific gravity of 0.534 (20 c), and a. Why isn't lithium. Lithium Is Gas Or Not.
From ul.org
What Are LithiumIon Batteries? UL Research Institutes Lithium Is Gas Or Not Lithium is atomic number 3 on the periodic table with element symbol li. I understand that it exhibits metallic bonding, but why wouldn't it form a diatomic covalent bond with itself like. Under the proper conditions, the element also combines with sulfur,. Here are a collection of lithium facts, including its properties, uses, and sources. Most lithium is currently produced. Lithium Is Gas Or Not.
From www.entrapeer.com
Pros and Cons of LithiumIon Batteries Entrapeer Lithium Is Gas Or Not I understand that it exhibits metallic bonding, but why wouldn't it form a diatomic covalent bond with itself like. Spodumene, petalite, lepidolite, and amblygonite are the more important minerals containing lithium. Pure lithium will form lithium hydroxide due to moisture in the air, as well as lithium nitride (\(li_3n\)) from \(n_2\) gas, and lithium carbonate \((li_2co_3\)) from carbon dioxide. Most. Lithium Is Gas Or Not.
From www.theodoregray.com
Facts, pictures, stories about the element Lithium in the Periodic Table Lithium Is Gas Or Not Here are a collection of lithium facts, including its properties, uses, and sources. I understand that it exhibits metallic bonding, but why wouldn't it form a diatomic covalent bond with itself like. Lithium does not react with oxygen at room temperature, but above 100°c does so to form lithium oxide (li 2 0). Under the proper conditions, the element also. Lithium Is Gas Or Not.
From chemistrypage.in
Lithium Element How LithiumIon Batteries Work Use of Lithium Lithium Is Gas Or Not Lithium does not react with oxygen at room temperature, but above 100°c does so to form lithium oxide (li 2 0). I understand that it exhibits metallic bonding, but why wouldn't it form a diatomic covalent bond with itself like. Lithium is atomic number 3 on the periodic table with element symbol li. Spodumene, petalite, lepidolite, and amblygonite are the. Lithium Is Gas Or Not.
From www.slideserve.com
PPT KS4 Chemistry PowerPoint Presentation, free download ID797217 Lithium Is Gas Or Not Why isn't lithium a gas? Pure lithium will form lithium hydroxide due to moisture in the air, as well as lithium nitride (\(li_3n\)) from \(n_2\) gas, and lithium carbonate \((li_2co_3\)) from carbon dioxide. Lithium has a melting point of 180.54 c, a boiling point of 1342 c, a specific gravity of 0.534 (20 c), and a. I understand that it. Lithium Is Gas Or Not.
From topblogtenz.com
Abbreviated electron configuration calculator [Noble gas] Lithium Is Gas Or Not Why isn't lithium a gas? Lithium has a melting point of 180.54 c, a boiling point of 1342 c, a specific gravity of 0.534 (20 c), and a. Here are a collection of lithium facts, including its properties, uses, and sources. I understand that it exhibits metallic bonding, but why wouldn't it form a diatomic covalent bond with itself like.. Lithium Is Gas Or Not.
From sustainablechronicles.com
Hydrogen gas cells vs. lithiumion batteries Powering EVs Lithium Is Gas Or Not Pure lithium will form lithium hydroxide due to moisture in the air, as well as lithium nitride (\(li_3n\)) from \(n_2\) gas, and lithium carbonate \((li_2co_3\)) from carbon dioxide. Lithium is atomic number 3 on the periodic table with element symbol li. Why isn't lithium a gas? Most lithium is currently produced in. I understand that it exhibits metallic bonding, but. Lithium Is Gas Or Not.
From www.youtube.com
How to Balance Li + H2 = LiH Lithium + Hydrogen gas (500700°C) YouTube Lithium Is Gas Or Not Under the proper conditions, the element also combines with sulfur,. Spodumene, petalite, lepidolite, and amblygonite are the more important minerals containing lithium. Lithium is atomic number 3 on the periodic table with element symbol li. I understand that it exhibits metallic bonding, but why wouldn't it form a diatomic covalent bond with itself like. Pure lithium will form lithium hydroxide. Lithium Is Gas Or Not.
From techiescientist.com
19 Amazing Uses of Lithium That You Must Know Techiescientist Lithium Is Gas Or Not Lithium does not react with oxygen at room temperature, but above 100°c does so to form lithium oxide (li 2 0). Spodumene, petalite, lepidolite, and amblygonite are the more important minerals containing lithium. Lithium has a melting point of 180.54 c, a boiling point of 1342 c, a specific gravity of 0.534 (20 c), and a. Pure lithium will form. Lithium Is Gas Or Not.
From mungfali.com
Lithium Ion Battery Anode And Cathode Lithium Is Gas Or Not Lithium has a melting point of 180.54 c, a boiling point of 1342 c, a specific gravity of 0.534 (20 c), and a. Pure lithium will form lithium hydroxide due to moisture in the air, as well as lithium nitride (\(li_3n\)) from \(n_2\) gas, and lithium carbonate \((li_2co_3\)) from carbon dioxide. Under the proper conditions, the element also combines with. Lithium Is Gas Or Not.
From www.vecteezy.com
Lithium symbol. Chemical element of the periodic table. Vector Lithium Is Gas Or Not Lithium is atomic number 3 on the periodic table with element symbol li. Lithium has a melting point of 180.54 c, a boiling point of 1342 c, a specific gravity of 0.534 (20 c), and a. Here are a collection of lithium facts, including its properties, uses, and sources. Pure lithium will form lithium hydroxide due to moisture in the. Lithium Is Gas Or Not.
From www.youtube.com
Gas vs Lithium YouTube Lithium Is Gas Or Not I understand that it exhibits metallic bonding, but why wouldn't it form a diatomic covalent bond with itself like. Lithium is atomic number 3 on the periodic table with element symbol li. Lithium does not react with oxygen at room temperature, but above 100°c does so to form lithium oxide (li 2 0). Pure lithium will form lithium hydroxide due. Lithium Is Gas Or Not.
From www.coursehero.com
[Solved] Look at the reaction between lithium and nitrogen (problem 3 Lithium Is Gas Or Not Most lithium is currently produced in. Lithium has a melting point of 180.54 c, a boiling point of 1342 c, a specific gravity of 0.534 (20 c), and a. Spodumene, petalite, lepidolite, and amblygonite are the more important minerals containing lithium. Why isn't lithium a gas? Lithium does not react with oxygen at room temperature, but above 100°c does so. Lithium Is Gas Or Not.
From www.pinterest.com
Lithium is a chemical element with the symbol Li and atomic number 3 Lithium Is Gas Or Not I understand that it exhibits metallic bonding, but why wouldn't it form a diatomic covalent bond with itself like. Here are a collection of lithium facts, including its properties, uses, and sources. Under the proper conditions, the element also combines with sulfur,. Lithium does not react with oxygen at room temperature, but above 100°c does so to form lithium oxide. Lithium Is Gas Or Not.
From www.chegg.com
Solved The reaction of lithium metal and water to form Lithium Is Gas Or Not Here are a collection of lithium facts, including its properties, uses, and sources. Lithium has a melting point of 180.54 c, a boiling point of 1342 c, a specific gravity of 0.534 (20 c), and a. Lithium does not react with oxygen at room temperature, but above 100°c does so to form lithium oxide (li 2 0). I understand that. Lithium Is Gas Or Not.
From discovermaterials.co.uk
Lithium ion (Liion) Batteries Discover Materials Lithium Is Gas Or Not Lithium does not react with oxygen at room temperature, but above 100°c does so to form lithium oxide (li 2 0). Here are a collection of lithium facts, including its properties, uses, and sources. Lithium has a melting point of 180.54 c, a boiling point of 1342 c, a specific gravity of 0.534 (20 c), and a. Lithium is atomic. Lithium Is Gas Or Not.
From als.lbl.gov
New Insights into LithiumMetal Surface Reactions for NextGeneration Lithium Is Gas Or Not Why isn't lithium a gas? I understand that it exhibits metallic bonding, but why wouldn't it form a diatomic covalent bond with itself like. Under the proper conditions, the element also combines with sulfur,. Spodumene, petalite, lepidolite, and amblygonite are the more important minerals containing lithium. Lithium has a melting point of 180.54 c, a boiling point of 1342 c,. Lithium Is Gas Or Not.
From ichca.com
Battery Safety Week Day 3 Allianz Risk Consulting bulletin Lithium Lithium Is Gas Or Not Under the proper conditions, the element also combines with sulfur,. Why isn't lithium a gas? Lithium is atomic number 3 on the periodic table with element symbol li. Lithium has a melting point of 180.54 c, a boiling point of 1342 c, a specific gravity of 0.534 (20 c), and a. Most lithium is currently produced in. Pure lithium will. Lithium Is Gas Or Not.
From www.thoughtco.com
First 20 Elements of the Periodic Table Lithium Is Gas Or Not Here are a collection of lithium facts, including its properties, uses, and sources. Lithium is atomic number 3 on the periodic table with element symbol li. Spodumene, petalite, lepidolite, and amblygonite are the more important minerals containing lithium. Lithium does not react with oxygen at room temperature, but above 100°c does so to form lithium oxide (li 2 0). Pure. Lithium Is Gas Or Not.
From owlcation.com
A Simple Comparison of Six LithiumIon Battery Types Owlcation Lithium Is Gas Or Not Under the proper conditions, the element also combines with sulfur,. Spodumene, petalite, lepidolite, and amblygonite are the more important minerals containing lithium. Lithium is atomic number 3 on the periodic table with element symbol li. Most lithium is currently produced in. I understand that it exhibits metallic bonding, but why wouldn't it form a diatomic covalent bond with itself like.. Lithium Is Gas Or Not.
From www.youtube.com
Li+N2=Li3N Balanced EquationLithium+Nitrogen=Lithium nitride Balanced Lithium Is Gas Or Not Pure lithium will form lithium hydroxide due to moisture in the air, as well as lithium nitride (\(li_3n\)) from \(n_2\) gas, and lithium carbonate \((li_2co_3\)) from carbon dioxide. I understand that it exhibits metallic bonding, but why wouldn't it form a diatomic covalent bond with itself like. Lithium has a melting point of 180.54 c, a boiling point of 1342. Lithium Is Gas Or Not.
From www.thoughtco.com
The Chemical and Physical Properties of Lithium, or Li Lithium Is Gas Or Not Lithium has a melting point of 180.54 c, a boiling point of 1342 c, a specific gravity of 0.534 (20 c), and a. Lithium is atomic number 3 on the periodic table with element symbol li. Lithium does not react with oxygen at room temperature, but above 100°c does so to form lithium oxide (li 2 0). Spodumene, petalite, lepidolite,. Lithium Is Gas Or Not.
From www.slideserve.com
PPT Lithium PowerPoint Presentation, free download ID5231803 Lithium Is Gas Or Not I understand that it exhibits metallic bonding, but why wouldn't it form a diatomic covalent bond with itself like. Spodumene, petalite, lepidolite, and amblygonite are the more important minerals containing lithium. Lithium is atomic number 3 on the periodic table with element symbol li. Under the proper conditions, the element also combines with sulfur,. Lithium does not react with oxygen. Lithium Is Gas Or Not.
From www.dreamstime.com
Lithium Chemical Periodic Element Icon. Vector Chemistry Lithium Sign Lithium Is Gas Or Not Lithium does not react with oxygen at room temperature, but above 100°c does so to form lithium oxide (li 2 0). Under the proper conditions, the element also combines with sulfur,. I understand that it exhibits metallic bonding, but why wouldn't it form a diatomic covalent bond with itself like. Most lithium is currently produced in. Pure lithium will form. Lithium Is Gas Or Not.
From hyetlithium.com
Solidstate Liion batteries HyET Lithium Lithium Is Gas Or Not Most lithium is currently produced in. Here are a collection of lithium facts, including its properties, uses, and sources. I understand that it exhibits metallic bonding, but why wouldn't it form a diatomic covalent bond with itself like. Lithium has a melting point of 180.54 c, a boiling point of 1342 c, a specific gravity of 0.534 (20 c), and. Lithium Is Gas Or Not.
From periodictableguide.com
Lithium (Li) Periodic Table (Element Information & More) Lithium Is Gas Or Not Here are a collection of lithium facts, including its properties, uses, and sources. I understand that it exhibits metallic bonding, but why wouldn't it form a diatomic covalent bond with itself like. Spodumene, petalite, lepidolite, and amblygonite are the more important minerals containing lithium. Why isn't lithium a gas? Lithium has a melting point of 180.54 c, a boiling point. Lithium Is Gas Or Not.
From synergyfiles.com
5 Types of Lithium Ion Batteries Lithium Is Gas Or Not I understand that it exhibits metallic bonding, but why wouldn't it form a diatomic covalent bond with itself like. Here are a collection of lithium facts, including its properties, uses, and sources. Under the proper conditions, the element also combines with sulfur,. Lithium is atomic number 3 on the periodic table with element symbol li. Lithium has a melting point. Lithium Is Gas Or Not.
From www.visualcapitalist.com
Infographic Lithium is the Fuel of the Green Revolution Lithium Is Gas Or Not Lithium has a melting point of 180.54 c, a boiling point of 1342 c, a specific gravity of 0.534 (20 c), and a. Spodumene, petalite, lepidolite, and amblygonite are the more important minerals containing lithium. Pure lithium will form lithium hydroxide due to moisture in the air, as well as lithium nitride (\(li_3n\)) from \(n_2\) gas, and lithium carbonate \((li_2co_3\)). Lithium Is Gas Or Not.
From www.britannica.com
lithium Definition, Properties, Use, & Facts Britannica Lithium Is Gas Or Not Lithium has a melting point of 180.54 c, a boiling point of 1342 c, a specific gravity of 0.534 (20 c), and a. Why isn't lithium a gas? Spodumene, petalite, lepidolite, and amblygonite are the more important minerals containing lithium. Here are a collection of lithium facts, including its properties, uses, and sources. Lithium does not react with oxygen at. Lithium Is Gas Or Not.
From sites.google.com
Lithium Table of Elements by Shrenil Sharma Lithium Is Gas Or Not Why isn't lithium a gas? Under the proper conditions, the element also combines with sulfur,. I understand that it exhibits metallic bonding, but why wouldn't it form a diatomic covalent bond with itself like. Lithium has a melting point of 180.54 c, a boiling point of 1342 c, a specific gravity of 0.534 (20 c), and a. Pure lithium will. Lithium Is Gas Or Not.
From www.nagwa.com
Question Video Molecular Formula of Lithium Oxide Nagwa Lithium Is Gas Or Not Lithium has a melting point of 180.54 c, a boiling point of 1342 c, a specific gravity of 0.534 (20 c), and a. Lithium is atomic number 3 on the periodic table with element symbol li. Under the proper conditions, the element also combines with sulfur,. Lithium does not react with oxygen at room temperature, but above 100°c does so. Lithium Is Gas Or Not.
From www.batterypowertips.com
Liion batteries, Part 2 cathodes Battery Power Tips Lithium Is Gas Or Not Why isn't lithium a gas? Pure lithium will form lithium hydroxide due to moisture in the air, as well as lithium nitride (\(li_3n\)) from \(n_2\) gas, and lithium carbonate \((li_2co_3\)) from carbon dioxide. Lithium has a melting point of 180.54 c, a boiling point of 1342 c, a specific gravity of 0.534 (20 c), and a. Lithium is atomic number. Lithium Is Gas Or Not.