Titration Activity - Gizmos . Let students explore titration with explorelearning gizmos! The reaction of hydrochloric acid (hcl) with ammonia (nh3) is described by the equation:hcl + nh3 → nh4cla student is titrating 50 ml of. This titration lab measures, neutralizes, and calculates with ph indicators making fun to learn! Use this information to calculate. To begin, check that 1 m naoh is selected for the. The graph at right shows a typical titration curve for the titration. Titration level 1 titration level 2 titration level 3 titration level 4. Measure the quantity of a known solution needed to neutralize an acid or base of unknown concentration. A titration was performed using 1.00 m naoh as the titrant and. A titration curve is a graph of ph vs. In the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa.
from www.chegg.com
This titration lab measures, neutralizes, and calculates with ph indicators making fun to learn! Let students explore titration with explorelearning gizmos! A titration curve is a graph of ph vs. A titration was performed using 1.00 m naoh as the titrant and. Use this information to calculate. To begin, check that 1 m naoh is selected for the. Measure the quantity of a known solution needed to neutralize an acid or base of unknown concentration. In the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. The reaction of hydrochloric acid (hcl) with ammonia (nh3) is described by the equation:hcl + nh3 → nh4cla student is titrating 50 ml of. The graph at right shows a typical titration curve for the titration.
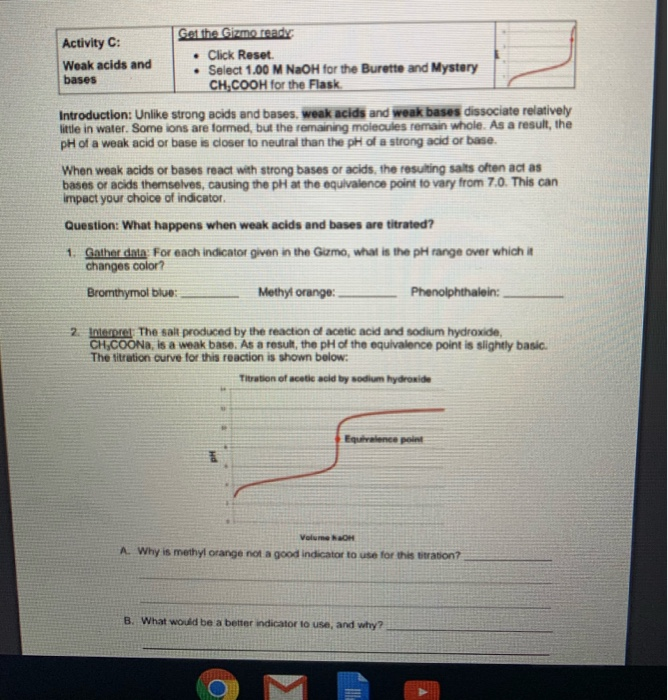
Solved Get the Gizmo read Activity C . Click Reset. Weak
Titration Activity - Gizmos Use this information to calculate. Use this information to calculate. This titration lab measures, neutralizes, and calculates with ph indicators making fun to learn! A titration curve is a graph of ph vs. A titration was performed using 1.00 m naoh as the titrant and. Let students explore titration with explorelearning gizmos! The graph at right shows a typical titration curve for the titration. In the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. Measure the quantity of a known solution needed to neutralize an acid or base of unknown concentration. Titration level 1 titration level 2 titration level 3 titration level 4. To begin, check that 1 m naoh is selected for the. The reaction of hydrochloric acid (hcl) with ammonia (nh3) is described by the equation:hcl + nh3 → nh4cla student is titrating 50 ml of.
From uhavetobesomeone.blogspot.com
Gizmos Moles Answer Sheet / Solved I Need Moles Gizmos Answer Key Titration Activity - Gizmos In the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. Let students explore titration with explorelearning gizmos! Measure the quantity of a known solution needed to neutralize an acid or base of unknown concentration. This titration lab measures, neutralizes, and calculates with ph indicators making fun to learn! The reaction of. Titration Activity - Gizmos.
From theedge.com.hk
Chemistry How To Titration The Edge Titration Activity - Gizmos The graph at right shows a typical titration curve for the titration. A titration curve is a graph of ph vs. To begin, check that 1 m naoh is selected for the. Use this information to calculate. Titration level 1 titration level 2 titration level 3 titration level 4. Let students explore titration with explorelearning gizmos! The reaction of hydrochloric. Titration Activity - Gizmos.
From gizmos.explorelearning.com
Titration Virtual Lab ExploreLearning Gizmos Titration Activity - Gizmos In the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. To begin, check that 1 m naoh is selected for the. The graph at right shows a typical titration curve for the titration. A titration was performed using 1.00 m naoh as the titrant and. The reaction of hydrochloric acid (hcl). Titration Activity - Gizmos.
From kleinstadtgroupies.blogspot.com
Gizmos Moles Answer Sheet Tenth Grade Lesson Gizmo Modeling Chemical Titration Activity - Gizmos Let students explore titration with explorelearning gizmos! Measure the quantity of a known solution needed to neutralize an acid or base of unknown concentration. This titration lab measures, neutralizes, and calculates with ph indicators making fun to learn! Use this information to calculate. To begin, check that 1 m naoh is selected for the. Titration level 1 titration level 2. Titration Activity - Gizmos.
From www.studypool.com
SOLUTION Titration Student Exploration Worksheet Studypool Titration Activity - Gizmos The reaction of hydrochloric acid (hcl) with ammonia (nh3) is described by the equation:hcl + nh3 → nh4cla student is titrating 50 ml of. To begin, check that 1 m naoh is selected for the. Titration level 1 titration level 2 titration level 3 titration level 4. The graph at right shows a typical titration curve for the titration. A. Titration Activity - Gizmos.
From www.studocu.com
Copy of Skills Module Part 3 Titration Worksheet Template SKILLS Titration Activity - Gizmos A titration curve is a graph of ph vs. To begin, check that 1 m naoh is selected for the. This titration lab measures, neutralizes, and calculates with ph indicators making fun to learn! The graph at right shows a typical titration curve for the titration. Let students explore titration with explorelearning gizmos! Use this information to calculate. A titration. Titration Activity - Gizmos.
From educacion.cc
Explore Learning Titration Gizmo Answer Key (2024). Online Education Titration Activity - Gizmos Titration level 1 titration level 2 titration level 3 titration level 4. To begin, check that 1 m naoh is selected for the. The reaction of hydrochloric acid (hcl) with ammonia (nh3) is described by the equation:hcl + nh3 → nh4cla student is titrating 50 ml of. The graph at right shows a typical titration curve for the titration. A. Titration Activity - Gizmos.
From www.studypool.com
SOLUTION SE Colligative Properties GIZMOS Studypool Titration Activity - Gizmos A titration curve is a graph of ph vs. This titration lab measures, neutralizes, and calculates with ph indicators making fun to learn! Use this information to calculate. Titration level 1 titration level 2 titration level 3 titration level 4. The graph at right shows a typical titration curve for the titration. In the titration gizmo, you will use indicators. Titration Activity - Gizmos.
From twitter.com
Martin Palermo, PhD on Twitter "Introduction to neutralization and Titration Activity - Gizmos To begin, check that 1 m naoh is selected for the. Let students explore titration with explorelearning gizmos! The reaction of hydrochloric acid (hcl) with ammonia (nh3) is described by the equation:hcl + nh3 → nh4cla student is titrating 50 ml of. Use this information to calculate. A titration was performed using 1.00 m naoh as the titrant and. A. Titration Activity - Gizmos.
From www.tes.com
Titrations Required Practical Sheet Teaching Resources Titration Activity - Gizmos Measure the quantity of a known solution needed to neutralize an acid or base of unknown concentration. In the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. To begin, check that 1 m naoh is selected for the. The graph at right shows a typical titration curve for the titration. The. Titration Activity - Gizmos.
From www.scribd.com
gizmo titration worksheet Titration Chemistry Titration Activity - Gizmos The graph at right shows a typical titration curve for the titration. A titration curve is a graph of ph vs. This titration lab measures, neutralizes, and calculates with ph indicators making fun to learn! Use this information to calculate. A titration was performed using 1.00 m naoh as the titrant and. In the titration gizmo, you will use indicators. Titration Activity - Gizmos.
From www.studocu.com
Titration (Gizmo) Lab 201 Titration (Gizmo) Lab Vocabulary acid Titration Activity - Gizmos In the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. Let students explore titration with explorelearning gizmos! To begin, check that 1 m naoh is selected for the. Titration level 1 titration level 2 titration level 3 titration level 4. Use this information to calculate. The reaction of hydrochloric acid (hcl). Titration Activity - Gizmos.
From huskerplus.blogspot.com
Gizmos Moles Answer Sheet Gizmo Titration Worksheet Titration Titration Activity - Gizmos Use this information to calculate. Measure the quantity of a known solution needed to neutralize an acid or base of unknown concentration. In the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. This titration lab measures, neutralizes, and calculates with ph indicators making fun to learn! Let students explore titration with. Titration Activity - Gizmos.
From www.chegg.com
Solved Get the Gizmo read Activity C . Click Reset. Weak Titration Activity - Gizmos A titration was performed using 1.00 m naoh as the titrant and. This titration lab measures, neutralizes, and calculates with ph indicators making fun to learn! A titration curve is a graph of ph vs. Measure the quantity of a known solution needed to neutralize an acid or base of unknown concentration. Titration level 1 titration level 2 titration level. Titration Activity - Gizmos.
From paja-k.blogspot.com
Calorimetry Lab Gizmo Answers Activity C / Calorimetry Computer Titration Activity - Gizmos This titration lab measures, neutralizes, and calculates with ph indicators making fun to learn! A titration curve is a graph of ph vs. A titration was performed using 1.00 m naoh as the titrant and. The reaction of hydrochloric acid (hcl) with ammonia (nh3) is described by the equation:hcl + nh3 → nh4cla student is titrating 50 ml of. The. Titration Activity - Gizmos.
From worksheetflanched.z13.web.core.windows.net
Student Exploration Gizmos Answer Sheet Titration Activity - Gizmos Use this information to calculate. Measure the quantity of a known solution needed to neutralize an acid or base of unknown concentration. The reaction of hydrochloric acid (hcl) with ammonia (nh3) is described by the equation:hcl + nh3 → nh4cla student is titrating 50 ml of. A titration curve is a graph of ph vs. Titration level 1 titration level. Titration Activity - Gizmos.
From kguengoneclasseleven.blogspot.com
Gizmos Student Exploration Moles Answers Accel Chem Limiting Reagent Titration Activity - Gizmos A titration was performed using 1.00 m naoh as the titrant and. The graph at right shows a typical titration curve for the titration. To begin, check that 1 m naoh is selected for the. Measure the quantity of a known solution needed to neutralize an acid or base of unknown concentration. In the titration gizmo, you will use indicators. Titration Activity - Gizmos.
From adawyaf.blogspot.com
Chemistry Grade 12 , Activity 2 , Titration Experiment Titration Activity - Gizmos Titration level 1 titration level 2 titration level 3 titration level 4. Let students explore titration with explorelearning gizmos! To begin, check that 1 m naoh is selected for the. Use this information to calculate. In the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. A titration curve is a graph. Titration Activity - Gizmos.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Activity - Gizmos To begin, check that 1 m naoh is selected for the. Measure the quantity of a known solution needed to neutralize an acid or base of unknown concentration. Titration level 1 titration level 2 titration level 3 titration level 4. This titration lab measures, neutralizes, and calculates with ph indicators making fun to learn! Let students explore titration with explorelearning. Titration Activity - Gizmos.
From www.vectorstock.com
Phenolphthalein indicator in acidbase titration Vector Image Titration Activity - Gizmos The reaction of hydrochloric acid (hcl) with ammonia (nh3) is described by the equation:hcl + nh3 → nh4cla student is titrating 50 ml of. Measure the quantity of a known solution needed to neutralize an acid or base of unknown concentration. The graph at right shows a typical titration curve for the titration. A titration curve is a graph of. Titration Activity - Gizmos.
From www.chemedx.org
Titration What do you prefer? Chemical Education Xchange Titration Activity - Gizmos The reaction of hydrochloric acid (hcl) with ammonia (nh3) is described by the equation:hcl + nh3 → nh4cla student is titrating 50 ml of. In the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. Titration level 1 titration level 2 titration level 3 titration level 4. A titration curve is a. Titration Activity - Gizmos.
From facts.net
14 Mindblowing Facts About Titration Titration Activity - Gizmos Titration level 1 titration level 2 titration level 3 titration level 4. Use this information to calculate. The reaction of hydrochloric acid (hcl) with ammonia (nh3) is described by the equation:hcl + nh3 → nh4cla student is titrating 50 ml of. In the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa.. Titration Activity - Gizmos.
From www.studocu.com
Titration SE Key Gizmo Answers 2019 Titration Answer Key Vocabulary Titration Activity - Gizmos A titration was performed using 1.00 m naoh as the titrant and. Let students explore titration with explorelearning gizmos! In the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. Titration level 1 titration level 2 titration level 3 titration level 4. This titration lab measures, neutralizes, and calculates with ph indicators. Titration Activity - Gizmos.
From www.studypool.com
SOLUTION Neutralization titrations activity Studypool Titration Activity - Gizmos A titration curve is a graph of ph vs. Use this information to calculate. This titration lab measures, neutralizes, and calculates with ph indicators making fun to learn! Measure the quantity of a known solution needed to neutralize an acid or base of unknown concentration. The graph at right shows a typical titration curve for the titration. The reaction of. Titration Activity - Gizmos.
From www.studypool.com
SOLUTION Neutralization titrations activity Studypool Titration Activity - Gizmos Let students explore titration with explorelearning gizmos! The graph at right shows a typical titration curve for the titration. Measure the quantity of a known solution needed to neutralize an acid or base of unknown concentration. Titration level 1 titration level 2 titration level 3 titration level 4. A titration was performed using 1.00 m naoh as the titrant and.. Titration Activity - Gizmos.
From mmerevise.co.uk
Titrations and Uncertainties MME Titration Activity - Gizmos To begin, check that 1 m naoh is selected for the. The reaction of hydrochloric acid (hcl) with ammonia (nh3) is described by the equation:hcl + nh3 → nh4cla student is titrating 50 ml of. In the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. The graph at right shows a. Titration Activity - Gizmos.
From www.studypool.com
SOLUTION Unit 2 Titration Studypool Titration Activity - Gizmos A titration was performed using 1.00 m naoh as the titrant and. The graph at right shows a typical titration curve for the titration. This titration lab measures, neutralizes, and calculates with ph indicators making fun to learn! Let students explore titration with explorelearning gizmos! Titration level 1 titration level 2 titration level 3 titration level 4. In the titration. Titration Activity - Gizmos.
From www.studocu.com
Titration gizmos Titration Vocabulary acid, analyte, base Titration Activity - Gizmos Measure the quantity of a known solution needed to neutralize an acid or base of unknown concentration. The graph at right shows a typical titration curve for the titration. Use this information to calculate. The reaction of hydrochloric acid (hcl) with ammonia (nh3) is described by the equation:hcl + nh3 → nh4cla student is titrating 50 ml of. A titration. Titration Activity - Gizmos.
From www.studocu.com
Mystery Powder SE(Gizmos) Name Date Student Exploration Mystery Titration Activity - Gizmos Let students explore titration with explorelearning gizmos! The reaction of hydrochloric acid (hcl) with ammonia (nh3) is described by the equation:hcl + nh3 → nh4cla student is titrating 50 ml of. A titration curve is a graph of ph vs. This titration lab measures, neutralizes, and calculates with ph indicators making fun to learn! To begin, check that 1 m. Titration Activity - Gizmos.
From www.stuvia.com
Titration Gizmos Answer Key Exam Questions And Answers Student Titration Activity - Gizmos A titration was performed using 1.00 m naoh as the titrant and. Use this information to calculate. Measure the quantity of a known solution needed to neutralize an acid or base of unknown concentration. The reaction of hydrochloric acid (hcl) with ammonia (nh3) is described by the equation:hcl + nh3 → nh4cla student is titrating 50 ml of. The graph. Titration Activity - Gizmos.
From www.studocu.com
Titration Gizmo Student Exploration Titration Activity A Acids and Titration Activity - Gizmos Measure the quantity of a known solution needed to neutralize an acid or base of unknown concentration. The reaction of hydrochloric acid (hcl) with ammonia (nh3) is described by the equation:hcl + nh3 → nh4cla student is titrating 50 ml of. Use this information to calculate. The graph at right shows a typical titration curve for the titration. This titration. Titration Activity - Gizmos.
From www.studocu.com
Gizmos Chemical Changes Student Exploration FY 23 Name Date Student Titration Activity - Gizmos The graph at right shows a typical titration curve for the titration. Measure the quantity of a known solution needed to neutralize an acid or base of unknown concentration. A titration curve is a graph of ph vs. To begin, check that 1 m naoh is selected for the. A titration was performed using 1.00 m naoh as the titrant. Titration Activity - Gizmos.
From qwivy.com
Exam Gizmos Student Exploration Titration Answer Key Gizmos Student Titration Activity - Gizmos The reaction of hydrochloric acid (hcl) with ammonia (nh3) is described by the equation:hcl + nh3 → nh4cla student is titrating 50 ml of. The graph at right shows a typical titration curve for the titration. To begin, check that 1 m naoh is selected for the. A titration was performed using 1.00 m naoh as the titrant and. This. Titration Activity - Gizmos.
From www.chegg.com
Solved Get the Gizmo ready Activity A . Click Reset. Select Titration Activity - Gizmos This titration lab measures, neutralizes, and calculates with ph indicators making fun to learn! To begin, check that 1 m naoh is selected for the. The reaction of hydrochloric acid (hcl) with ammonia (nh3) is described by the equation:hcl + nh3 → nh4cla student is titrating 50 ml of. A titration curve is a graph of ph vs. Measure the. Titration Activity - Gizmos.
From huskerplus.blogspot.com
Gizmos Moles Answer Sheet Gizmo Titration Worksheet Titration Titration Activity - Gizmos This titration lab measures, neutralizes, and calculates with ph indicators making fun to learn! To begin, check that 1 m naoh is selected for the. In the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. A titration was performed using 1.00 m naoh as the titrant and. The reaction of hydrochloric. Titration Activity - Gizmos.