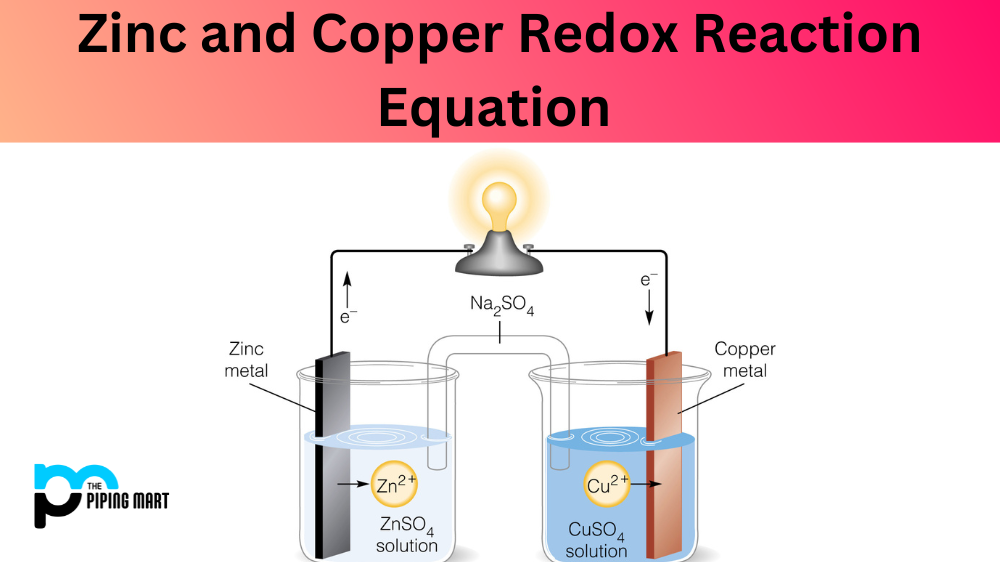
Copper And Zinc Equation . Magnesium is oxidised close oxidise chemical substances. Zn + cuso4 = cu + znso4 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole of aqueous. Just like water flowing spontaneously downhill, which can be made to do work by forcing a waterwheel, the flow of electrons from a higher potential energy to a lower one can also be harnessed to perform work. The solution gradually acquires the blue color. A simple redox reaction occurs when copper metal is immersed in a solution of silver nitrate. In this demonstration, dissolved copper ions come in contact with zinc, and zinc gives up its electrons to the copper. Hence electrons flow spontaneously from zinc to copper(ii) ions, forming zinc(ii) ions and metallic copper. Zinc loses two of its. When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip begins to darken. Cu + zno = cuo + zn is a single displacement (substitution) reaction where one mole of copper [cu] and one mole of zinc oxide [zno] react to. Magnesium + copper(ii) oxide → magnesium oxide + copper in this reaction: If left in the solution for a longer period of time, the zinc will gradually decay due to oxidation to zinc ions. Oxidation/reduction reactions between metals and metal ions.
from blog.thepipingmart.com
A simple redox reaction occurs when copper metal is immersed in a solution of silver nitrate. Oxidation/reduction reactions between metals and metal ions. Zinc loses two of its. Hence electrons flow spontaneously from zinc to copper(ii) ions, forming zinc(ii) ions and metallic copper. Magnesium is oxidised close oxidise chemical substances. If left in the solution for a longer period of time, the zinc will gradually decay due to oxidation to zinc ions. In this demonstration, dissolved copper ions come in contact with zinc, and zinc gives up its electrons to the copper. Zn + cuso4 = cu + znso4 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole of aqueous. Magnesium + copper(ii) oxide → magnesium oxide + copper in this reaction: The solution gradually acquires the blue color.
Zinc and Copper Redox Reaction Equation
Copper And Zinc Equation Cu + zno = cuo + zn is a single displacement (substitution) reaction where one mole of copper [cu] and one mole of zinc oxide [zno] react to. If left in the solution for a longer period of time, the zinc will gradually decay due to oxidation to zinc ions. Magnesium is oxidised close oxidise chemical substances. In this demonstration, dissolved copper ions come in contact with zinc, and zinc gives up its electrons to the copper. Magnesium + copper(ii) oxide → magnesium oxide + copper in this reaction: Zinc loses two of its. Cu + zno = cuo + zn is a single displacement (substitution) reaction where one mole of copper [cu] and one mole of zinc oxide [zno] react to. Zn + cuso4 = cu + znso4 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole of aqueous. The solution gradually acquires the blue color. A simple redox reaction occurs when copper metal is immersed in a solution of silver nitrate. Just like water flowing spontaneously downhill, which can be made to do work by forcing a waterwheel, the flow of electrons from a higher potential energy to a lower one can also be harnessed to perform work. Hence electrons flow spontaneously from zinc to copper(ii) ions, forming zinc(ii) ions and metallic copper. Oxidation/reduction reactions between metals and metal ions. When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip begins to darken.
From www.gauthmath.com
The equation 0.95c+0.05n=8.87 represents the density of a copperzinc Copper And Zinc Equation Just like water flowing spontaneously downhill, which can be made to do work by forcing a waterwheel, the flow of electrons from a higher potential energy to a lower one can also be harnessed to perform work. Cu + zno = cuo + zn is a single displacement (substitution) reaction where one mole of copper [cu] and one mole of. Copper And Zinc Equation.
From www.slideserve.com
PPT Cells and Voltage PowerPoint Presentation ID5231819 Copper And Zinc Equation A simple redox reaction occurs when copper metal is immersed in a solution of silver nitrate. Magnesium + copper(ii) oxide → magnesium oxide + copper in this reaction: In this demonstration, dissolved copper ions come in contact with zinc, and zinc gives up its electrons to the copper. Hence electrons flow spontaneously from zinc to copper(ii) ions, forming zinc(ii) ions. Copper And Zinc Equation.
From schoolworkhelper.net
Single Displacement Reactions Lab Explained SchoolWorkHelper Copper And Zinc Equation In this demonstration, dissolved copper ions come in contact with zinc, and zinc gives up its electrons to the copper. Hence electrons flow spontaneously from zinc to copper(ii) ions, forming zinc(ii) ions and metallic copper. The solution gradually acquires the blue color. A simple redox reaction occurs when copper metal is immersed in a solution of silver nitrate. Magnesium +. Copper And Zinc Equation.
From blog.thepipingmart.com
Difference Between Copper and Zinc Copper And Zinc Equation A simple redox reaction occurs when copper metal is immersed in a solution of silver nitrate. If left in the solution for a longer period of time, the zinc will gradually decay due to oxidation to zinc ions. Zinc loses two of its. Oxidation/reduction reactions between metals and metal ions. The solution gradually acquires the blue color. In this demonstration,. Copper And Zinc Equation.
From slideplayer.com
Chemical Reactions & Equations ppt download Copper And Zinc Equation Just like water flowing spontaneously downhill, which can be made to do work by forcing a waterwheel, the flow of electrons from a higher potential energy to a lower one can also be harnessed to perform work. If left in the solution for a longer period of time, the zinc will gradually decay due to oxidation to zinc ions. Magnesium. Copper And Zinc Equation.
From express.adobe.com
Zinc and Copper Chloride Copper And Zinc Equation Magnesium + copper(ii) oxide → magnesium oxide + copper in this reaction: Oxidation/reduction reactions between metals and metal ions. A simple redox reaction occurs when copper metal is immersed in a solution of silver nitrate. When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc. Copper And Zinc Equation.
From www.coursehero.com
[Solved] Sketch a diagram of a copper/zinc Daniell cell. Label all the Copper And Zinc Equation In this demonstration, dissolved copper ions come in contact with zinc, and zinc gives up its electrons to the copper. If left in the solution for a longer period of time, the zinc will gradually decay due to oxidation to zinc ions. Oxidation/reduction reactions between metals and metal ions. Just like water flowing spontaneously downhill, which can be made to. Copper And Zinc Equation.
From www.slideshare.net
Redox= quiz part 1 with answers Copper And Zinc Equation Hence electrons flow spontaneously from zinc to copper(ii) ions, forming zinc(ii) ions and metallic copper. If left in the solution for a longer period of time, the zinc will gradually decay due to oxidation to zinc ions. A simple redox reaction occurs when copper metal is immersed in a solution of silver nitrate. Just like water flowing spontaneously downhill, which. Copper And Zinc Equation.
From classnotes.org.in
Extraction of Copper and Zinc Chemistry, Class 12, General Principles Copper And Zinc Equation Zn + cuso4 = cu + znso4 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole of aqueous. Magnesium + copper(ii) oxide → magnesium oxide + copper in this reaction: Just like water flowing spontaneously downhill, which can be made to do work by forcing a waterwheel, the flow of electrons from a. Copper And Zinc Equation.
From www.chegg.com
Solved What is the net ionic equation for the reaction of Copper And Zinc Equation A simple redox reaction occurs when copper metal is immersed in a solution of silver nitrate. Magnesium is oxidised close oxidise chemical substances. The solution gradually acquires the blue color. Hence electrons flow spontaneously from zinc to copper(ii) ions, forming zinc(ii) ions and metallic copper. When a strip of zinc metal is placed into a blue solution of copper (ii). Copper And Zinc Equation.
From blog.thepipingmart.com
Classifications of Metals Copper vs. Zinc Copper And Zinc Equation The solution gradually acquires the blue color. When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip begins to darken. Cu + zno = cuo + zn is a single displacement (substitution) reaction where one mole of copper [cu] and one mole of zinc. Copper And Zinc Equation.
From www.researchgate.net
Phase diagram of the copperzinc system [17]. Download Scientific Diagram Copper And Zinc Equation If left in the solution for a longer period of time, the zinc will gradually decay due to oxidation to zinc ions. Magnesium + copper(ii) oxide → magnesium oxide + copper in this reaction: In this demonstration, dissolved copper ions come in contact with zinc, and zinc gives up its electrons to the copper. When a strip of zinc metal. Copper And Zinc Equation.
From hxejkrhaj.blob.core.windows.net
Copper 2 Sulfate Zinc Equation at Viola Smith blog Copper And Zinc Equation In this demonstration, dissolved copper ions come in contact with zinc, and zinc gives up its electrons to the copper. Oxidation/reduction reactions between metals and metal ions. Hence electrons flow spontaneously from zinc to copper(ii) ions, forming zinc(ii) ions and metallic copper. Zinc loses two of its. The solution gradually acquires the blue color. Cu + zno = cuo +. Copper And Zinc Equation.
From www.chegg.com
Solved (6pts) Reaction A Zinc Metal, Zn(s), and Copper(II) Copper And Zinc Equation Magnesium is oxidised close oxidise chemical substances. Hence electrons flow spontaneously from zinc to copper(ii) ions, forming zinc(ii) ions and metallic copper. In this demonstration, dissolved copper ions come in contact with zinc, and zinc gives up its electrons to the copper. The solution gradually acquires the blue color. Magnesium + copper(ii) oxide → magnesium oxide + copper in this. Copper And Zinc Equation.
From byjus.com
A piece of brass (alloy of zinc and copper) weighs 12.9 gm in air. When Copper And Zinc Equation Just like water flowing spontaneously downhill, which can be made to do work by forcing a waterwheel, the flow of electrons from a higher potential energy to a lower one can also be harnessed to perform work. Magnesium is oxidised close oxidise chemical substances. The solution gradually acquires the blue color. Cu + zno = cuo + zn is a. Copper And Zinc Equation.
From www.numerade.com
SOLVED The reaction between zinc and copper sulphate is illustrated Copper And Zinc Equation Magnesium + copper(ii) oxide → magnesium oxide + copper in this reaction: Oxidation/reduction reactions between metals and metal ions. Just like water flowing spontaneously downhill, which can be made to do work by forcing a waterwheel, the flow of electrons from a higher potential energy to a lower one can also be harnessed to perform work. Magnesium is oxidised close. Copper And Zinc Equation.
From keplarllp.com
😀 Zn cuso4 net ionic equation. Help writing complete ionic and net Copper And Zinc Equation Just like water flowing spontaneously downhill, which can be made to do work by forcing a waterwheel, the flow of electrons from a higher potential energy to a lower one can also be harnessed to perform work. Magnesium is oxidised close oxidise chemical substances. Cu + zno = cuo + zn is a single displacement (substitution) reaction where one mole. Copper And Zinc Equation.
From blog.thepipingmart.com
A Breakdown of Copper vs. Zinc Reactivity Copper And Zinc Equation A simple redox reaction occurs when copper metal is immersed in a solution of silver nitrate. The solution gradually acquires the blue color. If left in the solution for a longer period of time, the zinc will gradually decay due to oxidation to zinc ions. Oxidation/reduction reactions between metals and metal ions. Zinc loses two of its. Magnesium is oxidised. Copper And Zinc Equation.
From blog.thepipingmart.com
Zinc and Copper Redox Reaction Equation Copper And Zinc Equation Cu + zno = cuo + zn is a single displacement (substitution) reaction where one mole of copper [cu] and one mole of zinc oxide [zno] react to. Oxidation/reduction reactions between metals and metal ions. Magnesium is oxidised close oxidise chemical substances. If left in the solution for a longer period of time, the zinc will gradually decay due to. Copper And Zinc Equation.
From www.youtube.com
2. The ratio of copper and zinc in brass is 13 7. How much zinc will Copper And Zinc Equation Oxidation/reduction reactions between metals and metal ions. Zn + cuso4 = cu + znso4 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole of aqueous. Magnesium is oxidised close oxidise chemical substances. When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately. Copper And Zinc Equation.
From www.pastpapersinside.com
Redox Reaction Past Papers Inside Copper And Zinc Equation Hence electrons flow spontaneously from zinc to copper(ii) ions, forming zinc(ii) ions and metallic copper. Oxidation/reduction reactions between metals and metal ions. In this demonstration, dissolved copper ions come in contact with zinc, and zinc gives up its electrons to the copper. If left in the solution for a longer period of time, the zinc will gradually decay due to. Copper And Zinc Equation.
From www.meritnation.com
write a word equation for the reaction of Zinc with copper sulphate Copper And Zinc Equation Zn + cuso4 = cu + znso4 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole of aqueous. Hence electrons flow spontaneously from zinc to copper(ii) ions, forming zinc(ii) ions and metallic copper. Just like water flowing spontaneously downhill, which can be made to do work by forcing a waterwheel, the flow of. Copper And Zinc Equation.
From www.slideshare.net
Replacement reactions Copper And Zinc Equation Oxidation/reduction reactions between metals and metal ions. If left in the solution for a longer period of time, the zinc will gradually decay due to oxidation to zinc ions. A simple redox reaction occurs when copper metal is immersed in a solution of silver nitrate. Just like water flowing spontaneously downhill, which can be made to do work by forcing. Copper And Zinc Equation.
From www.coursehero.com
[Solved] Copper Observation Balanced Chemical Equation Zinc Observation Copper And Zinc Equation If left in the solution for a longer period of time, the zinc will gradually decay due to oxidation to zinc ions. Magnesium is oxidised close oxidise chemical substances. The solution gradually acquires the blue color. Magnesium + copper(ii) oxide → magnesium oxide + copper in this reaction: Zinc loses two of its. When a strip of zinc metal is. Copper And Zinc Equation.
From www.numerade.com
SOLVED Write the Lewis Dot Structure of Copper Sulfate and Zinc Copper And Zinc Equation Zn + cuso4 = cu + znso4 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole of aqueous. Cu + zno = cuo + zn is a single displacement (substitution) reaction where one mole of copper [cu] and one mole of zinc oxide [zno] react to. A simple redox reaction occurs when copper. Copper And Zinc Equation.
From www.numerade.com
SOLVED Write a balanced equation for the singlereplacement oxidation Copper And Zinc Equation The solution gradually acquires the blue color. Just like water flowing spontaneously downhill, which can be made to do work by forcing a waterwheel, the flow of electrons from a higher potential energy to a lower one can also be harnessed to perform work. Zinc loses two of its. Oxidation/reduction reactions between metals and metal ions. Cu + zno =. Copper And Zinc Equation.
From www.nagwa.com
Question Video Calculating the Standard Cell Potential for a Copper Copper And Zinc Equation Magnesium + copper(ii) oxide → magnesium oxide + copper in this reaction: Zinc loses two of its. When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip begins to darken. Zn + cuso4 = cu + znso4 is a single displacement (substitution) reaction where. Copper And Zinc Equation.
From www.slideshare.net
Redox= quiz part 1 with answers Copper And Zinc Equation In this demonstration, dissolved copper ions come in contact with zinc, and zinc gives up its electrons to the copper. Zn + cuso4 = cu + znso4 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole of aqueous. Magnesium is oxidised close oxidise chemical substances. When a strip of zinc metal is placed. Copper And Zinc Equation.
From www.youtube.com
What happens when a zinc strip is dipped into a copper sulphate Copper And Zinc Equation If left in the solution for a longer period of time, the zinc will gradually decay due to oxidation to zinc ions. Oxidation/reduction reactions between metals and metal ions. Zinc loses two of its. Cu + zno = cuo + zn is a single displacement (substitution) reaction where one mole of copper [cu] and one mole of zinc oxide [zno]. Copper And Zinc Equation.
From www.chegg.com
Solved 761. Zinc metal reacts with aqueous copper(II) Copper And Zinc Equation Cu + zno = cuo + zn is a single displacement (substitution) reaction where one mole of copper [cu] and one mole of zinc oxide [zno] react to. In this demonstration, dissolved copper ions come in contact with zinc, and zinc gives up its electrons to the copper. Magnesium is oxidised close oxidise chemical substances. A simple redox reaction occurs. Copper And Zinc Equation.
From brainly.in
An alloy is to contain copper and zinc in the ratio 9 4. The zinc Copper And Zinc Equation The solution gradually acquires the blue color. In this demonstration, dissolved copper ions come in contact with zinc, and zinc gives up its electrons to the copper. Hence electrons flow spontaneously from zinc to copper(ii) ions, forming zinc(ii) ions and metallic copper. Magnesium is oxidised close oxidise chemical substances. When a strip of zinc metal is placed into a blue. Copper And Zinc Equation.
From www.youtube.com
How to Balance Zn + CuCl2 = ZnCl2 + Cu Zinc + Copper (II) chloride Copper And Zinc Equation Cu + zno = cuo + zn is a single displacement (substitution) reaction where one mole of copper [cu] and one mole of zinc oxide [zno] react to. Oxidation/reduction reactions between metals and metal ions. Zn + cuso4 = cu + znso4 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole of aqueous.. Copper And Zinc Equation.
From hxejkrhaj.blob.core.windows.net
Copper 2 Sulfate Zinc Equation at Viola Smith blog Copper And Zinc Equation Magnesium is oxidised close oxidise chemical substances. The solution gradually acquires the blue color. Oxidation/reduction reactions between metals and metal ions. Hence electrons flow spontaneously from zinc to copper(ii) ions, forming zinc(ii) ions and metallic copper. When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the. Copper And Zinc Equation.
From www.coursehero.com
[Solved] For the reaction between zinc and copper(II) sulfate, describe Copper And Zinc Equation A simple redox reaction occurs when copper metal is immersed in a solution of silver nitrate. Oxidation/reduction reactions between metals and metal ions. In this demonstration, dissolved copper ions come in contact with zinc, and zinc gives up its electrons to the copper. Magnesium + copper(ii) oxide → magnesium oxide + copper in this reaction: When a strip of zinc. Copper And Zinc Equation.
From www.youtube.com
Net Ionic Equation for Zn + CuSO4 Zinc + Copper (II) Sulfate YouTube Copper And Zinc Equation In this demonstration, dissolved copper ions come in contact with zinc, and zinc gives up its electrons to the copper. The solution gradually acquires the blue color. Oxidation/reduction reactions between metals and metal ions. When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip. Copper And Zinc Equation.