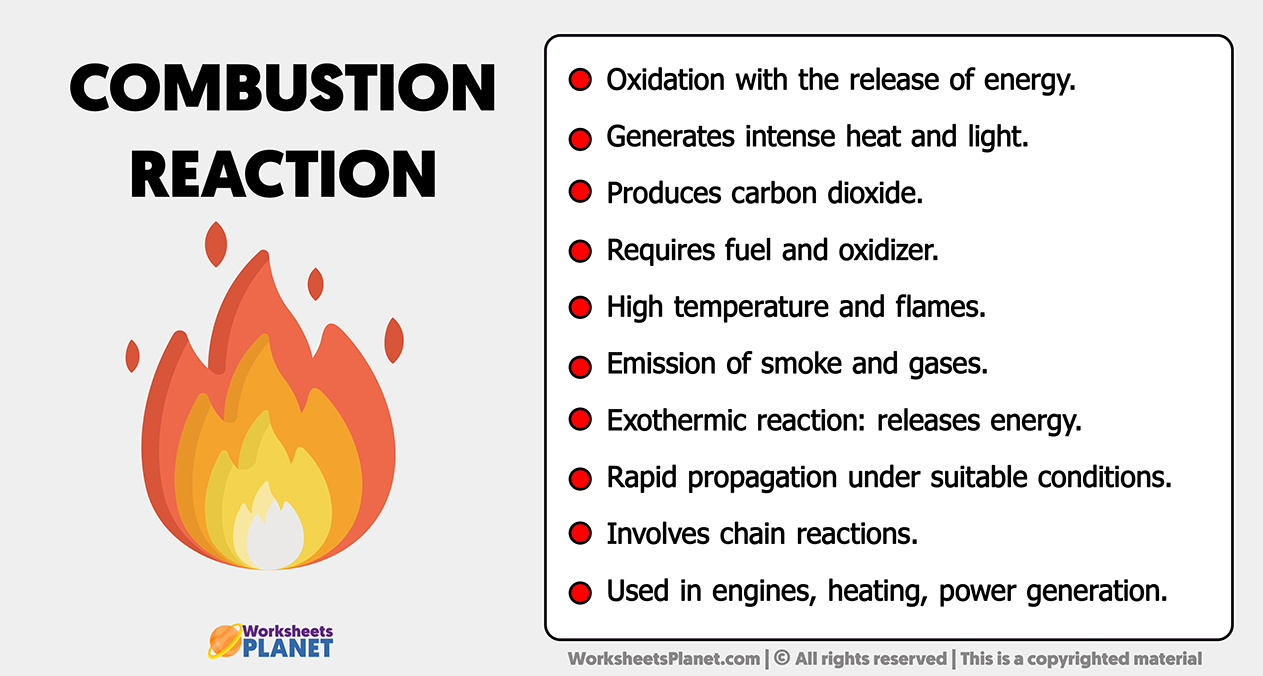
Combustion Reaction Produces . A fuel is any compound which has stored energy. 2 h 2 (g) + o 2 (g) → 2 h 2 o (g) A combustion reaction is an exothermic chemical reaction between substances, usually including oxygen gas and accompanied by the generation of heat, energy, and light (flame). Combustion reactions involve o 2 as one reactant. What is a combustion reaction? The combustion of hydrogen gas producing water vapor qualifies as a combustion reaction: A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. The products of a combustion reaction depend on the combusted substance. Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the generation of heat and light in the form of flame. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. Combustion is burning a fuel in oxygen, which gives out heat.
from www.worksheetsplanet.com
A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. Combustion reactions involve o 2 as one reactant. Combustion is burning a fuel in oxygen, which gives out heat. A fuel is any compound which has stored energy. What is a combustion reaction? The combustion of hydrogen gas producing water vapor qualifies as a combustion reaction: The products of a combustion reaction depend on the combusted substance. Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the generation of heat and light in the form of flame. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. 2 h 2 (g) + o 2 (g) → 2 h 2 o (g)
Combustion Reaction Characteristics
Combustion Reaction Produces The combustion of hydrogen gas producing water vapor qualifies as a combustion reaction: Combustion reactions involve o 2 as one reactant. A fuel is any compound which has stored energy. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the generation of heat and light in the form of flame. 2 h 2 (g) + o 2 (g) → 2 h 2 o (g) What is a combustion reaction? The products of a combustion reaction depend on the combusted substance. A combustion reaction is an exothermic chemical reaction between substances, usually including oxygen gas and accompanied by the generation of heat, energy, and light (flame). Combustion is burning a fuel in oxygen, which gives out heat. The combustion of hydrogen gas producing water vapor qualifies as a combustion reaction: A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat.
From www.slideserve.com
PPT Combustion Reactions PowerPoint Presentation, free download ID Combustion Reaction Produces A combustion reaction is an exothermic chemical reaction between substances, usually including oxygen gas and accompanied by the generation of heat, energy, and light (flame). The combustion of hydrogen gas producing water vapor qualifies as a combustion reaction: A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and. Combustion Reaction Produces.
From study.com
Predicting the Products of a Combustion Reaction Chemistry Combustion Reaction Produces A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the generation of heat and light in the form of flame. A combustion reaction is an exothermic chemical reaction between substances, usually including oxygen. Combustion Reaction Produces.
From sciencenotes.org
Combustion Reaction Definition and Examples Combustion Reaction Produces Combustion is burning a fuel in oxygen, which gives out heat. What is a combustion reaction? The combustion of hydrogen gas producing water vapor qualifies as a combustion reaction: A fuel is any compound which has stored energy. 2 h 2 (g) + o 2 (g) → 2 h 2 o (g) A combustion reaction is a reaction in which. Combustion Reaction Produces.
From www.youtube.com
Combustion Reactions YouTube Combustion Reaction Produces Combustion reactions involve o 2 as one reactant. What is a combustion reaction? 2 h 2 (g) + o 2 (g) → 2 h 2 o (g) The products of a combustion reaction depend on the combusted substance. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and. Combustion Reaction Produces.
From www.chemistrylearner.com
Combustion Reaction Definition, Characteristics & Examples Combustion Reaction Produces Combustion is burning a fuel in oxygen, which gives out heat. A fuel is any compound which has stored energy. Combustion reactions involve o 2 as one reactant. The products of a combustion reaction depend on the combusted substance. What is a combustion reaction? A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy. Combustion Reaction Produces.
From www.youtube.com
Examples of Combustion Reactions YouTube Combustion Reaction Produces Combustion reactions involve o 2 as one reactant. What is a combustion reaction? Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the generation of heat and light in the form of flame. Combustion is burning a fuel in oxygen, which gives out heat. A combustion reaction is a reaction in which a substance reacts with. Combustion Reaction Produces.
From www.wou.edu
CH150 Chapter 5 Chemical Reactions Chemistry Combustion Reaction Produces A fuel is any compound which has stored energy. A combustion reaction is an exothermic chemical reaction between substances, usually including oxygen gas and accompanied by the generation of heat, energy, and light (flame). 2 h 2 (g) + o 2 (g) → 2 h 2 o (g) What is a combustion reaction? Combustion reactions involve o 2 as one. Combustion Reaction Produces.
From www.chemistrystudent.com
Alkanes (ALevel) ChemistryStudent Combustion Reaction Produces Combustion is burning a fuel in oxygen, which gives out heat. Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the generation of heat and light in the form of flame. The products of a combustion reaction depend on the combusted substance. What is a combustion reaction? A combustion reaction is an exothermic chemical reaction between. Combustion Reaction Produces.
From slideplayer.com
Reaction Types Combustion Reactions. ppt download Combustion Reaction Produces A combustion reaction is an exothermic chemical reaction between substances, usually including oxygen gas and accompanied by the generation of heat, energy, and light (flame). 2 h 2 (g) + o 2 (g) → 2 h 2 o (g) Combustion reactions involve o 2 as one reactant. A combustion reaction is a reaction in which a substance reacts with oxygen. Combustion Reaction Produces.
From www.slideserve.com
PPT Combustion Reactions PowerPoint Presentation, free download ID Combustion Reaction Produces A fuel is any compound which has stored energy. Combustion is burning a fuel in oxygen, which gives out heat. The products of a combustion reaction depend on the combusted substance. The combustion of hydrogen gas producing water vapor qualifies as a combustion reaction: Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the generation of. Combustion Reaction Produces.
From www.thoughtco.com
What Is a Combustion Reaction? Definition and Examples Combustion Reaction Produces 2 h 2 (g) + o 2 (g) → 2 h 2 o (g) The products of a combustion reaction depend on the combusted substance. Combustion is burning a fuel in oxygen, which gives out heat. Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the generation of heat and light in the form of flame.. Combustion Reaction Produces.
From gavynyouthbooth.blogspot.com
Describe What Happens in a Combustion Reaction Combustion Reaction Produces A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the generation of heat and light in the form of flame. A fuel is any compound which has stored energy. 2 h 2 (g). Combustion Reaction Produces.
From www.slideserve.com
PPT Chapter 6 Chemical Reactions PowerPoint Presentation, free Combustion Reaction Produces A combustion reaction is an exothermic chemical reaction between substances, usually including oxygen gas and accompanied by the generation of heat, energy, and light (flame). A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. 2 h 2 (g) + o 2 (g) → 2 h 2. Combustion Reaction Produces.
From ar.inspiredpencil.com
Combustion Reaction Diagram Combustion Reaction Produces A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. Combustion reactions involve o 2 as one reactant. A fuel is any compound which has stored energy. The combustion of hydrogen gas producing water vapor qualifies as a combustion reaction: A combustion reaction is an exothermic chemical. Combustion Reaction Produces.
From fyorssrtn.blob.core.windows.net
Combustion Reaction Gasoline at Doris Vanburen blog Combustion Reaction Produces 2 h 2 (g) + o 2 (g) → 2 h 2 o (g) What is a combustion reaction? Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the generation of heat and light in the form of flame. Combustion is burning a fuel in oxygen, which gives out heat. Combustion reactions involve o 2 as. Combustion Reaction Produces.
From treatybottle13.pythonanywhere.com
Simple Combustion Reaction Octane Physics Formulas Of Class 12 Combustion Reaction Produces The combustion of hydrogen gas producing water vapor qualifies as a combustion reaction: 2 h 2 (g) + o 2 (g) → 2 h 2 o (g) A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. A combustion reaction is a reaction in which a substance. Combustion Reaction Produces.
From www.slideserve.com
PPT CHEMICAL REACTIONS AND EQUATIONS PowerPoint Presentation, free Combustion Reaction Produces The combustion of hydrogen gas producing water vapor qualifies as a combustion reaction: 2 h 2 (g) + o 2 (g) → 2 h 2 o (g) A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. Combustion is burning a fuel in oxygen, which gives out. Combustion Reaction Produces.
From www.shalom-education.com
Fuels and Combustion KS3 Chemistry Revision Combustion Reaction Produces Combustion reactions involve o 2 as one reactant. A combustion reaction is an exothermic chemical reaction between substances, usually including oxygen gas and accompanied by the generation of heat, energy, and light (flame). Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the generation of heat and light in the form of flame. The combustion of. Combustion Reaction Produces.
From www.expii.com
Combustion Reactions — Definition & Examples Expii Combustion Reaction Produces 2 h 2 (g) + o 2 (g) → 2 h 2 o (g) A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. Combustion reactions involve o 2 as one reactant. What is a combustion reaction? Combustion is burning a fuel in oxygen, which gives out. Combustion Reaction Produces.
From giojqvmqu.blob.core.windows.net
Describe A Combustion Reaction at Whitney Conrad blog Combustion Reaction Produces Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the generation of heat and light in the form of flame. Combustion reactions involve o 2 as one reactant. Combustion is burning a fuel in oxygen, which gives out heat. 2 h 2 (g) + o 2 (g) → 2 h 2 o (g) The products of. Combustion Reaction Produces.
From www.slideshare.net
combustion reactions Combustion Reaction Produces A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. Combustion reactions involve o 2 as one reactant. A combustion reaction is an exothermic chemical reaction between substances, usually including oxygen gas and accompanied by the generation of heat, energy, and light (flame). 2 h 2 (g). Combustion Reaction Produces.
From www.slideserve.com
PPT COMBUSTION REACTIONS PowerPoint Presentation, free download ID Combustion Reaction Produces What is a combustion reaction? A combustion reaction is an exothermic chemical reaction between substances, usually including oxygen gas and accompanied by the generation of heat, energy, and light (flame). Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the generation of heat and light in the form of flame. Combustion is burning a fuel in. Combustion Reaction Produces.
From sciencetallis.weebly.com
7. Organic Chemistry THOMAS TALLIS SCIENCE Combustion Reaction Produces Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the generation of heat and light in the form of flame. 2 h 2 (g) + o 2 (g) → 2 h 2 o (g) A fuel is any compound which has stored energy. A combustion reaction is a reaction in which a substance reacts with oxygen. Combustion Reaction Produces.
From slideplayer.com
Chapter ppt download Combustion Reaction Produces Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the generation of heat and light in the form of flame. Combustion is burning a fuel in oxygen, which gives out heat. What is a combustion reaction? Combustion reactions involve o 2 as one reactant. The combustion of hydrogen gas producing water vapor qualifies as a combustion. Combustion Reaction Produces.
From slideplayer.com
Chapter 9 Chemical Reactions ppt download Combustion Reaction Produces Combustion reactions involve o 2 as one reactant. 2 h 2 (g) + o 2 (g) → 2 h 2 o (g) Combustion is burning a fuel in oxygen, which gives out heat. A fuel is any compound which has stored energy. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the. Combustion Reaction Produces.
From www.chemicals.co.uk
What is Combustion in Chemistry? The Chemistry Blog Combustion Reaction Produces A fuel is any compound which has stored energy. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. What is a combustion reaction? Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the generation of heat and light in the form of. Combustion Reaction Produces.
From www.slideserve.com
PPT Combustion PowerPoint Presentation, free download ID1992427 Combustion Reaction Produces The products of a combustion reaction depend on the combusted substance. Combustion reactions involve o 2 as one reactant. What is a combustion reaction? Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the generation of heat and light in the form of flame. A fuel is any compound which has stored energy. Combustion is burning. Combustion Reaction Produces.
From www.worksheetsplanet.com
Combustion Reaction Characteristics Combustion Reaction Produces A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. A combustion reaction is an exothermic chemical reaction between substances, usually including oxygen gas and accompanied by the generation of heat, energy, and light (flame). Combustion is burning a fuel in oxygen, which gives out heat. The. Combustion Reaction Produces.
From slideplayer.com
Some general types of chemical reactions are ppt download Combustion Reaction Produces Combustion is burning a fuel in oxygen, which gives out heat. 2 h 2 (g) + o 2 (g) → 2 h 2 o (g) The combustion of hydrogen gas producing water vapor qualifies as a combustion reaction: A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and. Combustion Reaction Produces.
From www.youtube.com
Chemical Reactions (3 of 11) Combustion Reactions, An Explanation YouTube Combustion Reaction Produces A fuel is any compound which has stored energy. Combustion is burning a fuel in oxygen, which gives out heat. A combustion reaction is an exothermic chemical reaction between substances, usually including oxygen gas and accompanied by the generation of heat, energy, and light (flame). A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing. Combustion Reaction Produces.
From ar.inspiredpencil.com
Combustion Chemistry Combustion Reaction Produces What is a combustion reaction? Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the generation of heat and light in the form of flame. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. The products of a combustion reaction depend on. Combustion Reaction Produces.
From sciencetrends.com
Combustion Reaction Examples And Definition Science Trends Combustion Reaction Produces Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the generation of heat and light in the form of flame. The products of a combustion reaction depend on the combusted substance. 2 h 2 (g) + o 2 (g) → 2 h 2 o (g) A combustion reaction is a reaction in which a substance reacts. Combustion Reaction Produces.
From slideplayer.com
Chapter 8 Chemical Reaction ppt download Combustion Reaction Produces 2 h 2 (g) + o 2 (g) → 2 h 2 o (g) The products of a combustion reaction depend on the combusted substance. A fuel is any compound which has stored energy. Combustion is burning a fuel in oxygen, which gives out heat. What is a combustion reaction? Combustion, a chemical reaction between substances, usually including oxygen and. Combustion Reaction Produces.
From www.tes.com
Secondary chemistry teaching resources Chemical reactions TES Combustion Reaction Produces What is a combustion reaction? A combustion reaction is an exothermic chemical reaction between substances, usually including oxygen gas and accompanied by the generation of heat, energy, and light (flame). A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. A combustion reaction is a reaction in. Combustion Reaction Produces.
From www.slideserve.com
PPT Combustion PowerPoint Presentation, free download ID5525141 Combustion Reaction Produces A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. A combustion reaction is an exothermic chemical reaction between substances, usually including oxygen gas and accompanied by the generation of heat, energy, and light (flame). What is a combustion reaction? Combustion reactions involve o 2 as one. Combustion Reaction Produces.