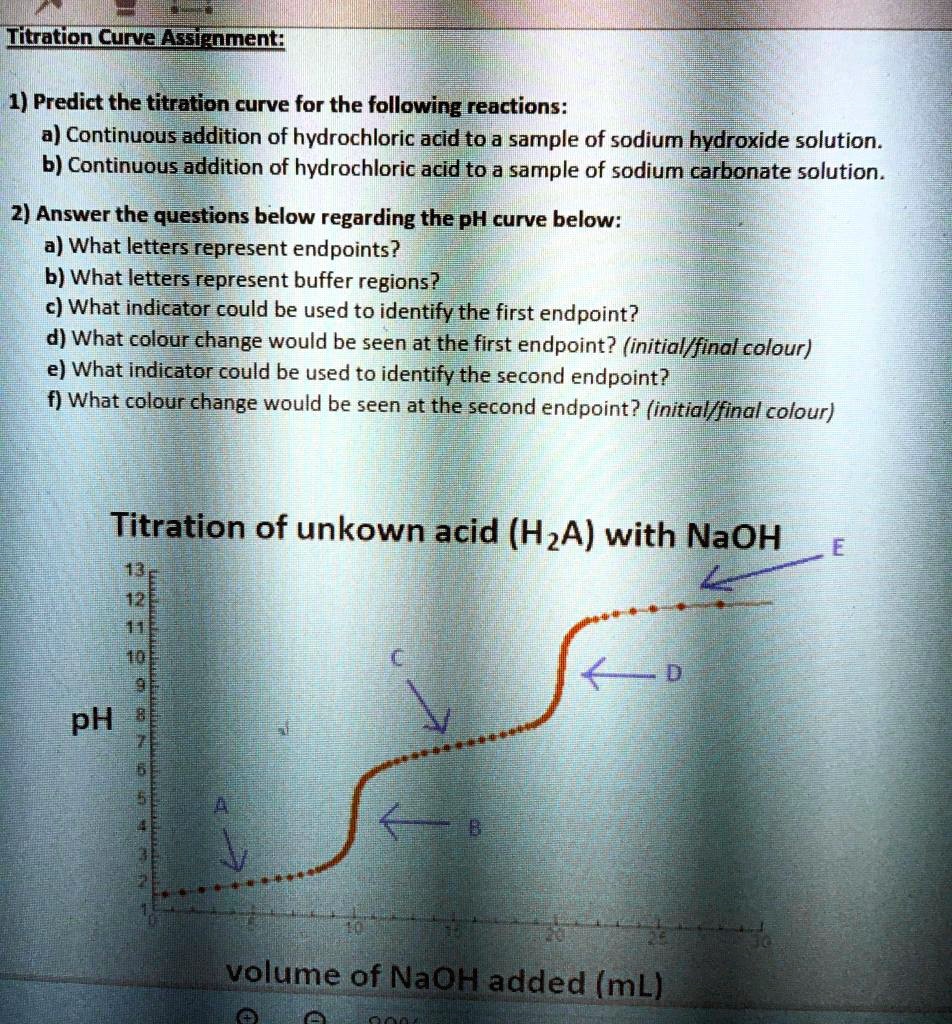
End Point In Titration Definition . A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. This process continues until stoichiometrically equivalent amounts of the reactants have been mixed, and an endpoint known as the equivalence point has been reached. The endpoint is the observable point in a titration where an indicator signals that the reaction has completed, while the equivalence point is the. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: Using a ph probe and using a colour indicator. A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. The point in a titration where. There are many different types of titrations that differ by the titrant used and substances that can be. Definitions of titrant, titration curve, end point and equivalence point. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the analyte. The point in a titration where the indicator changes color, indicating the completion of the reaction. The completion of a titration is the end point, detected by some type of physical change produced by the solution, such as a color.
from www.animalia-life.club
Definitions of titrant, titration curve, end point and equivalence point. The point in a titration where. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the analyte. A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. The completion of a titration is the end point, detected by some type of physical change produced by the solution, such as a color. The endpoint is the observable point in a titration where an indicator signals that the reaction has completed, while the equivalence point is the. There are many different types of titrations that differ by the titrant used and substances that can be. This process continues until stoichiometrically equivalent amounts of the reactants have been mixed, and an endpoint known as the equivalence point has been reached. Using a ph probe and using a colour indicator. The point in a titration where the indicator changes color, indicating the completion of the reaction.
Endpoint Titration
End Point In Titration Definition This process continues until stoichiometrically equivalent amounts of the reactants have been mixed, and an endpoint known as the equivalence point has been reached. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the analyte. A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. This process continues until stoichiometrically equivalent amounts of the reactants have been mixed, and an endpoint known as the equivalence point has been reached. The point in a titration where the indicator changes color, indicating the completion of the reaction. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: The point in a titration where. Using a ph probe and using a colour indicator. The completion of a titration is the end point, detected by some type of physical change produced by the solution, such as a color. The endpoint is the observable point in a titration where an indicator signals that the reaction has completed, while the equivalence point is the. There are many different types of titrations that differ by the titrant used and substances that can be. Definitions of titrant, titration curve, end point and equivalence point. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached.
From www.slideserve.com
PPT Chapter 14 Acids and Bases PowerPoint Presentation, free download End Point In Titration Definition The point in a titration where the indicator changes color, indicating the completion of the reaction. The endpoint is the observable point in a titration where an indicator signals that the reaction has completed, while the equivalence point is the. This process continues until stoichiometrically equivalent amounts of the reactants have been mixed, and an endpoint known as the equivalence. End Point In Titration Definition.
From www.animalia-life.club
Endpoint Titration End Point In Titration Definition A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. The point in a titration where the indicator changes color, indicating the completion of the reaction. Using a ph probe and using a colour indicator. The completion of. End Point In Titration Definition.
From www.youtube.com
Titration Definition, steps and formula YouTube End Point In Titration Definition A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. Definitions of titrant, titration curve, end point and equivalence point. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: There are many different types of titrations that. End Point In Titration Definition.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog End Point In Titration Definition Using a ph probe and using a colour indicator. This process continues until stoichiometrically equivalent amounts of the reactants have been mixed, and an endpoint known as the equivalence point has been reached. There are many different types of titrations that differ by the titrant used and substances that can be. A titration is a volumetric technique in which a. End Point In Titration Definition.
From www.ck12.org
Titration Curve Overview ( Video ) Chemistry CK12 Foundation End Point In Titration Definition Using a ph probe and using a colour indicator. The completion of a titration is the end point, detected by some type of physical change produced by the solution, such as a color. There are many different types of titrations that differ by the titrant used and substances that can be. A point of equivalence in a titration refers to. End Point In Titration Definition.
From narodnatribuna.info
Ppt How To Interpret Titration Curves Powerpoint End Point In Titration Definition The point in a titration where. A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: The point in a titration where the indicator changes color, indicating the. End Point In Titration Definition.
From www.chemistrylearner.com
Complexometric Titration Definition, Examples, and Applications End Point In Titration Definition The point in a titration where. There are many different types of titrations that differ by the titrant used and substances that can be. The point in a titration where the indicator changes color, indicating the completion of the reaction. The endpoint is the observable point in a titration where an indicator signals that the reaction has completed, while the. End Point In Titration Definition.
From letitsnowglobe.co.uk
Titration procedure pdf End Point In Titration Definition A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. Using a ph probe and using a colour indicator. There are many different types of titrations that differ by the titrant used and substances that can be. Titration. End Point In Titration Definition.
From www.studypool.com
SOLUTION Chemistry acid base titration Studypool End Point In Titration Definition The completion of a titration is the end point, detected by some type of physical change produced by the solution, such as a color. Definitions of titrant, titration curve, end point and equivalence point. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as. End Point In Titration Definition.
From www.slideserve.com
PPT Titrations PowerPoint Presentation, free download ID2976284 End Point In Titration Definition The completion of a titration is the end point, detected by some type of physical change produced by the solution, such as a color. The point in a titration where the indicator changes color, indicating the completion of the reaction. The endpoint is the observable point in a titration where an indicator signals that the reaction has completed, while the. End Point In Titration Definition.
From www.animalia-life.club
Endpoint Titration End Point In Titration Definition For a ph titration (between an acid and a base), there are two common ways to find the endpoint: A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. There are many different types of titrations that differ by the titrant used and substances that can be.. End Point In Titration Definition.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple End Point In Titration Definition There are many different types of titrations that differ by the titrant used and substances that can be. The point in a titration where. The point in a titration where the indicator changes color, indicating the completion of the reaction. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: The. End Point In Titration Definition.
From www.researchgate.net
The relation between alkalinity end point and pH titration curve. End End Point In Titration Definition There are many different types of titrations that differ by the titrant used and substances that can be. The endpoint is the observable point in a titration where an indicator signals that the reaction has completed, while the equivalence point is the. A point of equivalence in a titration refers to a point at which the added titrant is chemically. End Point In Titration Definition.
From www.youtube.com
What is the difference between the endpoint and equivalence point in a End Point In Titration Definition Using a ph probe and using a colour indicator. A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. End Point In Titration Definition.
From scienceinfo.com
Acidbase Titration 4 Types, Theory, Working Principle End Point In Titration Definition For a ph titration (between an acid and a base), there are two common ways to find the endpoint: A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. Using a ph probe and using a colour indicator.. End Point In Titration Definition.
From www.chemistrystudent.com
Titration Curves (ALevel) ChemistryStudent End Point In Titration Definition A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: There are many different types of titrations that differ by the titrant used and substances that can be.. End Point In Titration Definition.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation End Point In Titration Definition Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the analyte. Using a ph probe and using a colour indicator. There are many different types of titrations that differ by the titrant used and substances that can be. The endpoint is the observable. End Point In Titration Definition.
From ar.inspiredpencil.com
Endpoint Titration End Point In Titration Definition For a ph titration (between an acid and a base), there are two common ways to find the endpoint: The completion of a titration is the end point, detected by some type of physical change produced by the solution, such as a color. A point of equivalence in a titration refers to a point at which the added titrant is. End Point In Titration Definition.
From www.microlit.com
An Advanced Guide to Titration Microlit End Point In Titration Definition Definitions of titrant, titration curve, end point and equivalence point. Using a ph probe and using a colour indicator. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. A point of equivalence in a titration refers to. End Point In Titration Definition.
From www.osmosis.org
Introduction to titrations Vídeo & Anatomía Osmosis End Point In Titration Definition For a ph titration (between an acid and a base), there are two common ways to find the endpoint: A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. Definitions of titrant, titration curve, end point and equivalence. End Point In Titration Definition.
From www.animalia-life.club
Endpoint Titration End Point In Titration Definition The point in a titration where. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the analyte. The point in a titration where the indicator changes color, indicating the completion of the reaction. A titration is a volumetric technique in which a solution. End Point In Titration Definition.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators End Point In Titration Definition The point in a titration where the indicator changes color, indicating the completion of the reaction. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the analyte. A titration is a volumetric technique in which a solution of one reactant (the titrant) is. End Point In Titration Definition.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple End Point In Titration Definition Definitions of titrant, titration curve, end point and equivalence point. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the analyte. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a. End Point In Titration Definition.
From chem4three.blogspot.com
CHEMISTRY 11 TITRATIONS End Point In Titration Definition Using a ph probe and using a colour indicator. Definitions of titrant, titration curve, end point and equivalence point. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. The point in a titration where the indicator changes. End Point In Titration Definition.
From www.animalia-life.club
Endpoint Titration End Point In Titration Definition A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. Definitions of titrant, titration curve,. End Point In Titration Definition.
From courses.lumenlearning.com
AcidBase Titrations Chemistry Atoms First End Point In Titration Definition Definitions of titrant, titration curve, end point and equivalence point. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. The point in a titration where. A titration. End Point In Titration Definition.
From animalia-life.club
Endpoint Titration End Point In Titration Definition Definitions of titrant, titration curve, end point and equivalence point. A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. The point in a titration where. This process continues until stoichiometrically equivalent amounts of the reactants have been mixed, and an endpoint known as the equivalence point. End Point In Titration Definition.
From www.animalia-life.club
Endpoint Titration End Point In Titration Definition There are many different types of titrations that differ by the titrant used and substances that can be. Using a ph probe and using a colour indicator. This process continues until stoichiometrically equivalent amounts of the reactants have been mixed, and an endpoint known as the equivalence point has been reached. The endpoint is the observable point in a titration. End Point In Titration Definition.
From www.animalia-life.club
Endpoint Titration End Point In Titration Definition For a ph titration (between an acid and a base), there are two common ways to find the endpoint: Using a ph probe and using a colour indicator. A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. The point in a titration where the indicator changes. End Point In Titration Definition.
From theedge.com.hk
Chemistry How To Titration The Edge End Point In Titration Definition There are many different types of titrations that differ by the titrant used and substances that can be. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. The point in a titration where the indicator changes color,. End Point In Titration Definition.
From scienceinfo.com
Titration Definition, 4 Types, Procedure End Point In Titration Definition A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: The point in a titration where. The endpoint is. End Point In Titration Definition.
From differencesfinder.com
Difference Between Equivalence Point and End Point Differences Finder End Point In Titration Definition This process continues until stoichiometrically equivalent amounts of the reactants have been mixed, and an endpoint known as the equivalence point has been reached. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. The completion of a. End Point In Titration Definition.
From www.animalia-life.club
Endpoint Titration End Point In Titration Definition The point in a titration where the indicator changes color, indicating the completion of the reaction. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the analyte. A point of equivalence in a titration refers to a point at which the added titrant. End Point In Titration Definition.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts End Point In Titration Definition Definitions of titrant, titration curve, end point and equivalence point. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the analyte. The completion of a titration is the end point, detected by some type of physical change produced by the solution, such as. End Point In Titration Definition.
From www.vrogue.co
Titration 4 Redox Titration End Point Titration Scree vrogue.co End Point In Titration Definition A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. Using a ph probe and using a colour indicator. A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent. End Point In Titration Definition.