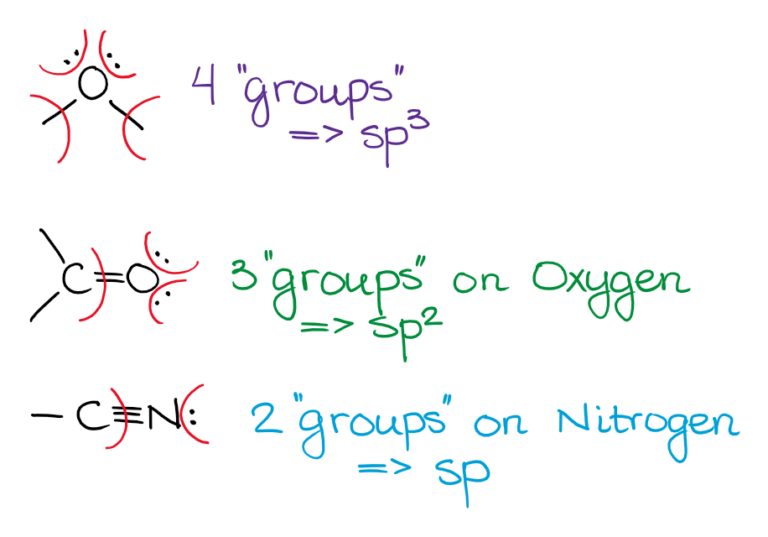Hybridization Of N In Hcn . Carbon is the central atom, so we can draw the skeletal structure: Carbon has a single bond with a hydrogen atom and a triple bond with a nitrogen atom. There is a total of 1 + 4 + 5 =. Here the hydrogen atom only needs one valence electron to attain a stable structure. Carbon undergoes sp hybridization, creating two equivalent sp hybrid orbitals that form σ bonds with h and n, and two. There is a simple formula that can be used to determine the hybridization of hcn easily, Hydrogen cyanide is made up of three atoms: The hybridization of hcn in sp. What is the hybridization of n in hcn? It is important to find the hybridization of any compound because it gives us an insight into how the electrons are distributed in different orbitals. Hybridization of hcn can be calculated using the following formula. This means only one of nitrogen’s p orbitals is available to be hybridized, and so the hybridization of nitrogen in hcn is “sp”. The lewis structure for hydrogen cyanide (hcn) involves representing the valence electrons of the constituent atoms in a way that.
from www.organicchemistrytutor.com
Hydrogen cyanide is made up of three atoms: Carbon undergoes sp hybridization, creating two equivalent sp hybrid orbitals that form σ bonds with h and n, and two. Carbon is the central atom, so we can draw the skeletal structure: There is a total of 1 + 4 + 5 =. What is the hybridization of n in hcn? Carbon has a single bond with a hydrogen atom and a triple bond with a nitrogen atom. Here the hydrogen atom only needs one valence electron to attain a stable structure. The lewis structure for hydrogen cyanide (hcn) involves representing the valence electrons of the constituent atoms in a way that. This means only one of nitrogen’s p orbitals is available to be hybridized, and so the hybridization of nitrogen in hcn is “sp”. It is important to find the hybridization of any compound because it gives us an insight into how the electrons are distributed in different orbitals.
Hybridization — Organic Chemistry Tutor
Hybridization Of N In Hcn Here the hydrogen atom only needs one valence electron to attain a stable structure. This means only one of nitrogen’s p orbitals is available to be hybridized, and so the hybridization of nitrogen in hcn is “sp”. There is a simple formula that can be used to determine the hybridization of hcn easily, Carbon is the central atom, so we can draw the skeletal structure: Here the hydrogen atom only needs one valence electron to attain a stable structure. The hybridization of hcn in sp. The lewis structure for hydrogen cyanide (hcn) involves representing the valence electrons of the constituent atoms in a way that. It is important to find the hybridization of any compound because it gives us an insight into how the electrons are distributed in different orbitals. Carbon undergoes sp hybridization, creating two equivalent sp hybrid orbitals that form σ bonds with h and n, and two. What is the hybridization of n in hcn? Carbon has a single bond with a hydrogen atom and a triple bond with a nitrogen atom. There is a total of 1 + 4 + 5 =. Hybridization of hcn can be calculated using the following formula. Hydrogen cyanide is made up of three atoms:
From courses.lumenlearning.com
13.1. Introduction Organic Chemistry II Hybridization Of N In Hcn The hybridization of hcn in sp. What is the hybridization of n in hcn? Carbon undergoes sp hybridization, creating two equivalent sp hybrid orbitals that form σ bonds with h and n, and two. Hydrogen cyanide is made up of three atoms: Here the hydrogen atom only needs one valence electron to attain a stable structure. This means only one. Hybridization Of N In Hcn.
From www.chegg.com
Solved QUESTION 4 Consider the HCN molecule (C is the Hybridization Of N In Hcn Here the hydrogen atom only needs one valence electron to attain a stable structure. Carbon undergoes sp hybridization, creating two equivalent sp hybrid orbitals that form σ bonds with h and n, and two. Hydrogen cyanide is made up of three atoms: Carbon has a single bond with a hydrogen atom and a triple bond with a nitrogen atom. There. Hybridization Of N In Hcn.
From www.chegg.com
Solved What is the hybridization of the indicated nitrogen Hybridization Of N In Hcn The lewis structure for hydrogen cyanide (hcn) involves representing the valence electrons of the constituent atoms in a way that. There is a simple formula that can be used to determine the hybridization of hcn easily, What is the hybridization of n in hcn? Hybridization of hcn can be calculated using the following formula. It is important to find the. Hybridization Of N In Hcn.
From ar.inspiredpencil.com
Hcn Hybridization Hybridization Of N In Hcn Carbon undergoes sp hybridization, creating two equivalent sp hybrid orbitals that form σ bonds with h and n, and two. What is the hybridization of n in hcn? The lewis structure for hydrogen cyanide (hcn) involves representing the valence electrons of the constituent atoms in a way that. The hybridization of hcn in sp. Carbon is the central atom, so. Hybridization Of N In Hcn.
From brainly.com
Determine the hybridization around the central atom for each of the Hybridization Of N In Hcn It is important to find the hybridization of any compound because it gives us an insight into how the electrons are distributed in different orbitals. There is a simple formula that can be used to determine the hybridization of hcn easily, The lewis structure for hydrogen cyanide (hcn) involves representing the valence electrons of the constituent atoms in a way. Hybridization Of N In Hcn.
From chem.libretexts.org
Hybridization Chemistry LibreTexts Hybridization Of N In Hcn Here the hydrogen atom only needs one valence electron to attain a stable structure. There is a simple formula that can be used to determine the hybridization of hcn easily, Hybridization of hcn can be calculated using the following formula. Carbon undergoes sp hybridization, creating two equivalent sp hybrid orbitals that form σ bonds with h and n, and two.. Hybridization Of N In Hcn.
From www.youtube.com
Depicting Hybridization of Atomic Orbitals Hydrogen Cyanide (HCN) 002 Hybridization Of N In Hcn There is a total of 1 + 4 + 5 =. Hydrogen cyanide is made up of three atoms: The lewis structure for hydrogen cyanide (hcn) involves representing the valence electrons of the constituent atoms in a way that. This means only one of nitrogen’s p orbitals is available to be hybridized, and so the hybridization of nitrogen in hcn. Hybridization Of N In Hcn.
From ar.inspiredpencil.com
Hcn Hybridization Hybridization Of N In Hcn The lewis structure for hydrogen cyanide (hcn) involves representing the valence electrons of the constituent atoms in a way that. Here the hydrogen atom only needs one valence electron to attain a stable structure. Hydrogen cyanide is made up of three atoms: What is the hybridization of n in hcn? It is important to find the hybridization of any compound. Hybridization Of N In Hcn.
From www.studypool.com
SOLUTION Hcn lewis structure molecular geometry hybridization polar or Hybridization Of N In Hcn What is the hybridization of n in hcn? There is a simple formula that can be used to determine the hybridization of hcn easily, Carbon has a single bond with a hydrogen atom and a triple bond with a nitrogen atom. It is important to find the hybridization of any compound because it gives us an insight into how the. Hybridization Of N In Hcn.
From www.chemistrylearner.com
Molecular Geometry, Lewis Structure, and Bond Angle of HCN Hybridization Of N In Hcn There is a simple formula that can be used to determine the hybridization of hcn easily, Carbon undergoes sp hybridization, creating two equivalent sp hybrid orbitals that form σ bonds with h and n, and two. What is the hybridization of n in hcn? The lewis structure for hydrogen cyanide (hcn) involves representing the valence electrons of the constituent atoms. Hybridization Of N In Hcn.
From www.organicchemistrytutor.com
Hybridization — Organic Chemistry Tutor Hybridization Of N In Hcn What is the hybridization of n in hcn? There is a simple formula that can be used to determine the hybridization of hcn easily, Here the hydrogen atom only needs one valence electron to attain a stable structure. This means only one of nitrogen’s p orbitals is available to be hybridized, and so the hybridization of nitrogen in hcn is. Hybridization Of N In Hcn.
From www.slideserve.com
PPT Orbital Hybridization PowerPoint Presentation, free download ID Hybridization Of N In Hcn Here the hydrogen atom only needs one valence electron to attain a stable structure. The lewis structure for hydrogen cyanide (hcn) involves representing the valence electrons of the constituent atoms in a way that. There is a total of 1 + 4 + 5 =. The hybridization of hcn in sp. Hybridization of hcn can be calculated using the following. Hybridization Of N In Hcn.
From www.youtube.com
Organic hybridization practice Chemical bonds Chemistry Khan Hybridization Of N In Hcn Here the hydrogen atom only needs one valence electron to attain a stable structure. There is a simple formula that can be used to determine the hybridization of hcn easily, Hydrogen cyanide is made up of three atoms: What is the hybridization of n in hcn? The hybridization of hcn in sp. It is important to find the hybridization of. Hybridization Of N In Hcn.
From www.slideserve.com
PPT sp Hybrid Orbitals We assume that the Be orbitals in the BeF Hybridization Of N In Hcn Hydrogen cyanide is made up of three atoms: Hybridization of hcn can be calculated using the following formula. There is a total of 1 + 4 + 5 =. The hybridization of hcn in sp. What is the hybridization of n in hcn? This means only one of nitrogen’s p orbitals is available to be hybridized, and so the hybridization. Hybridization Of N In Hcn.
From www.masterorganicchemistry.com
Bond Hybridization Practice Master Organic Chemistry Hybridization Of N In Hcn Carbon is the central atom, so we can draw the skeletal structure: What is the hybridization of n in hcn? The hybridization of hcn in sp. Hydrogen cyanide is made up of three atoms: Carbon undergoes sp hybridization, creating two equivalent sp hybrid orbitals that form σ bonds with h and n, and two. It is important to find the. Hybridization Of N In Hcn.
From www.slideserve.com
PPT Orbital Hybridization PowerPoint Presentation, free download ID Hybridization Of N In Hcn It is important to find the hybridization of any compound because it gives us an insight into how the electrons are distributed in different orbitals. There is a simple formula that can be used to determine the hybridization of hcn easily, Here the hydrogen atom only needs one valence electron to attain a stable structure. This means only one of. Hybridization Of N In Hcn.
From www.slideserve.com
PPT HYBRIDIZATION OF ATOMIC ORBITALS PowerPoint Presentation, free Hybridization Of N In Hcn Carbon is the central atom, so we can draw the skeletal structure: Here the hydrogen atom only needs one valence electron to attain a stable structure. Hybridization of hcn can be calculated using the following formula. There is a simple formula that can be used to determine the hybridization of hcn easily, Hydrogen cyanide is made up of three atoms:. Hybridization Of N In Hcn.
From www.youtube.com
Depicting Hybridization of Atomic Orbitals Hydrogen Cyanide (HCN) 001 Hybridization Of N In Hcn Here the hydrogen atom only needs one valence electron to attain a stable structure. Carbon has a single bond with a hydrogen atom and a triple bond with a nitrogen atom. Carbon is the central atom, so we can draw the skeletal structure: What is the hybridization of n in hcn? There is a simple formula that can be used. Hybridization Of N In Hcn.
From www.collegesearch.in
Types of Hybridization Definitions, Examples, Key Features, Steps to Hybridization Of N In Hcn There is a total of 1 + 4 + 5 =. Carbon undergoes sp hybridization, creating two equivalent sp hybrid orbitals that form σ bonds with h and n, and two. Hydrogen cyanide is made up of three atoms: It is important to find the hybridization of any compound because it gives us an insight into how the electrons are. Hybridization Of N In Hcn.
From www.chegg.com
Solved 22. Determine the hybridization of N in HCN Z of N = Hybridization Of N In Hcn What is the hybridization of n in hcn? It is important to find the hybridization of any compound because it gives us an insight into how the electrons are distributed in different orbitals. Here the hydrogen atom only needs one valence electron to attain a stable structure. Hydrogen cyanide is made up of three atoms: Carbon undergoes sp hybridization, creating. Hybridization Of N In Hcn.
From general.chemistrysteps.com
Hybridization of Atomic Orbitals Chemistry Steps Hybridization Of N In Hcn Hybridization of hcn can be calculated using the following formula. There is a total of 1 + 4 + 5 =. Hydrogen cyanide is made up of three atoms: Here the hydrogen atom only needs one valence electron to attain a stable structure. There is a simple formula that can be used to determine the hybridization of hcn easily, Carbon. Hybridization Of N In Hcn.
From pubs.acs.org
Variations in the Nature of Triple Bonds The N2, HCN, and HC2H Series Hybridization Of N In Hcn There is a total of 1 + 4 + 5 =. The lewis structure for hydrogen cyanide (hcn) involves representing the valence electrons of the constituent atoms in a way that. Carbon undergoes sp hybridization, creating two equivalent sp hybrid orbitals that form σ bonds with h and n, and two. Carbon is the central atom, so we can draw. Hybridization Of N In Hcn.
From www.numerade.com
SOLVED 1. Give the hybridization for the C and the O in H2CO Hybridization Of N In Hcn Hybridization of hcn can be calculated using the following formula. It is important to find the hybridization of any compound because it gives us an insight into how the electrons are distributed in different orbitals. Carbon has a single bond with a hydrogen atom and a triple bond with a nitrogen atom. Here the hydrogen atom only needs one valence. Hybridization Of N In Hcn.
From www.youtube.com
Hybridization of HCN YouTube Hybridization Of N In Hcn What is the hybridization of n in hcn? Carbon is the central atom, so we can draw the skeletal structure: Carbon has a single bond with a hydrogen atom and a triple bond with a nitrogen atom. It is important to find the hybridization of any compound because it gives us an insight into how the electrons are distributed in. Hybridization Of N In Hcn.
From byjus.com
hybridisation of N in 1.)H3C NO2 2.)H3C NH Hybridization Of N In Hcn Hydrogen cyanide is made up of three atoms: This means only one of nitrogen’s p orbitals is available to be hybridized, and so the hybridization of nitrogen in hcn is “sp”. Carbon is the central atom, so we can draw the skeletal structure: It is important to find the hybridization of any compound because it gives us an insight into. Hybridization Of N In Hcn.
From www.youtube.com
Hybridization of HCN molecule Chemistry Sciences YouTube Hybridization Of N In Hcn What is the hybridization of n in hcn? Here the hydrogen atom only needs one valence electron to attain a stable structure. Hydrogen cyanide is made up of three atoms: Carbon undergoes sp hybridization, creating two equivalent sp hybrid orbitals that form σ bonds with h and n, and two. It is important to find the hybridization of any compound. Hybridization Of N In Hcn.
From pubs.acs.org
Variations in the Nature of Triple Bonds The N2, HCN, and HC2H Series Hybridization Of N In Hcn The lewis structure for hydrogen cyanide (hcn) involves representing the valence electrons of the constituent atoms in a way that. What is the hybridization of n in hcn? There is a total of 1 + 4 + 5 =. Hybridization of hcn can be calculated using the following formula. The hybridization of hcn in sp. This means only one of. Hybridization Of N In Hcn.
From www.masterorganicchemistry.com
How To Determine Hybridization A Shortcut Master Organic Chemistry Hybridization Of N In Hcn The hybridization of hcn in sp. Hydrogen cyanide is made up of three atoms: Carbon undergoes sp hybridization, creating two equivalent sp hybrid orbitals that form σ bonds with h and n, and two. There is a total of 1 + 4 + 5 =. It is important to find the hybridization of any compound because it gives us an. Hybridization Of N In Hcn.
From ar.inspiredpencil.com
Hcn Hybridization Hybridization Of N In Hcn Hydrogen cyanide is made up of three atoms: Carbon is the central atom, so we can draw the skeletal structure: Carbon has a single bond with a hydrogen atom and a triple bond with a nitrogen atom. There is a total of 1 + 4 + 5 =. What is the hybridization of n in hcn? The lewis structure for. Hybridization Of N In Hcn.
From pubs.acs.org
Variations in the Nature of Triple Bonds The N2, HCN, and HC2H Series Hybridization Of N In Hcn Hydrogen cyanide is made up of three atoms: This means only one of nitrogen’s p orbitals is available to be hybridized, and so the hybridization of nitrogen in hcn is “sp”. There is a simple formula that can be used to determine the hybridization of hcn easily, There is a total of 1 + 4 + 5 =. What is. Hybridization Of N In Hcn.
From www.reddit.com
sp hybridization of nitrogen in HCN Hybridization Of N In Hcn There is a simple formula that can be used to determine the hybridization of hcn easily, The hybridization of hcn in sp. What is the hybridization of n in hcn? The lewis structure for hydrogen cyanide (hcn) involves representing the valence electrons of the constituent atoms in a way that. Carbon has a single bond with a hydrogen atom and. Hybridization Of N In Hcn.
From chemistryteory.blogspot.com
chemistry Hybridization In molecules containing double and triple bond. Hybridization Of N In Hcn Carbon has a single bond with a hydrogen atom and a triple bond with a nitrogen atom. It is important to find the hybridization of any compound because it gives us an insight into how the electrons are distributed in different orbitals. The lewis structure for hydrogen cyanide (hcn) involves representing the valence electrons of the constituent atoms in a. Hybridization Of N In Hcn.
From www.numerade.com
SOLVED N many of the following molecules have sp 2 hybridization on Hybridization Of N In Hcn It is important to find the hybridization of any compound because it gives us an insight into how the electrons are distributed in different orbitals. Hybridization of hcn can be calculated using the following formula. The hybridization of hcn in sp. There is a total of 1 + 4 + 5 =. Hydrogen cyanide is made up of three atoms:. Hybridization Of N In Hcn.
From mungfali.com
Lewis Dot Diagram For Hydrogen Hybridization Of N In Hcn The lewis structure for hydrogen cyanide (hcn) involves representing the valence electrons of the constituent atoms in a way that. It is important to find the hybridization of any compound because it gives us an insight into how the electrons are distributed in different orbitals. The hybridization of hcn in sp. Here the hydrogen atom only needs one valence electron. Hybridization Of N In Hcn.
From www.youtube.com
Hybridization of HCN (Hydrogen Cyanide) YouTube Hybridization Of N In Hcn There is a total of 1 + 4 + 5 =. This means only one of nitrogen’s p orbitals is available to be hybridized, and so the hybridization of nitrogen in hcn is “sp”. The hybridization of hcn in sp. What is the hybridization of n in hcn? There is a simple formula that can be used to determine the. Hybridization Of N In Hcn.