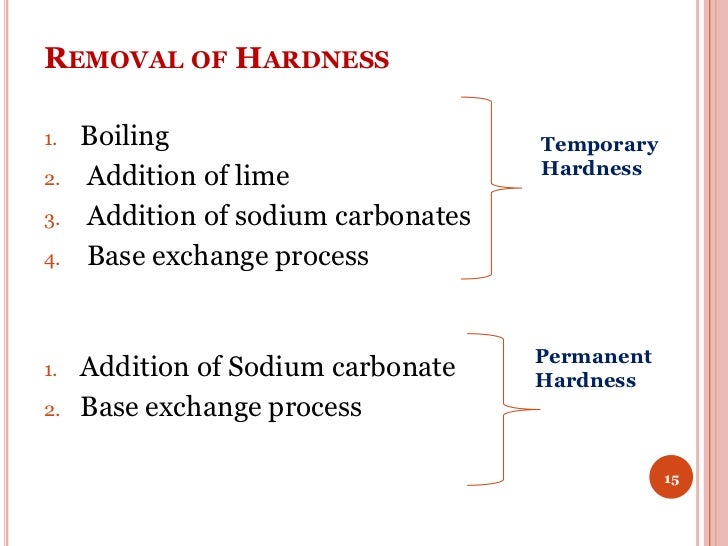
When Water Is Boiled The Hardness Removed Is Called . • when the water is boiled,. The presence of soluble bicarbonates, of calcium. Water that does not give lather with soap is called hard water. When temporary hard water is boiled, the soluble calcium hydrogencarbonate decomposes (breaks down) to form calcium. This method of removing hardness leads. Water that can have its hardness removed by boiling it is called temporary hard water. Boiling will remove cox2 c o x 2, making apparently cacox3 c a. In this case, the hardness in water can be removed by boiling the water. When we boil water, the soluble salts of mg(hco 3) 2 are converted to mg(oh) 2, which is insoluble, and hence gets precipitated and is removed. It reacts with soluble chlorides and. During boiling, bicarbonates of calcium and. (ii) the permanent hardness of water is removed when it is treated with a small quantity of sodium carbonate. • temporary hard water is caused by dissolved calcium hydrogencarbonate or magnesium hydrogencarbonate. Boiling will evaporate pure water, thus increasing the concentration of minerals including calcium, thereby increasing hardness. The temporary hardness of water can be removed by the following methods:
from www.slideshare.net
• when the water is boiled,. When we boil water, the soluble salts of mg(hco 3) 2 are converted to mg(oh) 2, which is insoluble, and hence gets precipitated and is removed. When temporary hard water is boiled, the soluble calcium hydrogencarbonate decomposes (breaks down) to form calcium. Water that does not give lather with soap is called hard water. It reacts with soluble chlorides and. The temporary hardness of water can be removed by the following methods: During boiling, bicarbonates of calcium and. The presence of soluble bicarbonates, of calcium. This method of removing hardness leads. Boiling will evaporate pure water, thus increasing the concentration of minerals including calcium, thereby increasing hardness.
water hardness
When Water Is Boiled The Hardness Removed Is Called Boiling will evaporate pure water, thus increasing the concentration of minerals including calcium, thereby increasing hardness. Boiling will remove cox2 c o x 2, making apparently cacox3 c a. • when the water is boiled,. Boiling will evaporate pure water, thus increasing the concentration of minerals including calcium, thereby increasing hardness. This method of removing hardness leads. Water that does not give lather with soap is called hard water. (ii) the permanent hardness of water is removed when it is treated with a small quantity of sodium carbonate. The presence of soluble bicarbonates, of calcium. Water that can have its hardness removed by boiling it is called temporary hard water. When we boil water, the soluble salts of mg(hco 3) 2 are converted to mg(oh) 2, which is insoluble, and hence gets precipitated and is removed. • temporary hard water is caused by dissolved calcium hydrogencarbonate or magnesium hydrogencarbonate. The temporary hardness of water can be removed by the following methods: In this case, the hardness in water can be removed by boiling the water. When temporary hard water is boiled, the soluble calcium hydrogencarbonate decomposes (breaks down) to form calcium. During boiling, bicarbonates of calcium and. It reacts with soluble chlorides and.
From www.slideserve.com
PPT Water Hardness PowerPoint Presentation, free download ID2529744 When Water Is Boiled The Hardness Removed Is Called It reacts with soluble chlorides and. When we boil water, the soluble salts of mg(hco 3) 2 are converted to mg(oh) 2, which is insoluble, and hence gets precipitated and is removed. The presence of soluble bicarbonates, of calcium. When temporary hard water is boiled, the soluble calcium hydrogencarbonate decomposes (breaks down) to form calcium. • temporary hard water is. When Water Is Boiled The Hardness Removed Is Called.
From en.ppt-online.org
Water and its properties online presentation When Water Is Boiled The Hardness Removed Is Called During boiling, bicarbonates of calcium and. It reacts with soluble chlorides and. In this case, the hardness in water can be removed by boiling the water. Boiling will evaporate pure water, thus increasing the concentration of minerals including calcium, thereby increasing hardness. • when the water is boiled,. The temporary hardness of water can be removed by the following methods:. When Water Is Boiled The Hardness Removed Is Called.
From www.slideserve.com
PPT Hardness of Water PowerPoint Presentation, free download ID6601427 When Water Is Boiled The Hardness Removed Is Called In this case, the hardness in water can be removed by boiling the water. During boiling, bicarbonates of calcium and. Boiling will evaporate pure water, thus increasing the concentration of minerals including calcium, thereby increasing hardness. Water that does not give lather with soap is called hard water. Boiling will remove cox2 c o x 2, making apparently cacox3 c. When Water Is Boiled The Hardness Removed Is Called.
From www.slideserve.com
PPT WATER A guide for GCSE students PowerPoint Presentation, free When Water Is Boiled The Hardness Removed Is Called • when the water is boiled,. It reacts with soluble chlorides and. This method of removing hardness leads. When temporary hard water is boiled, the soluble calcium hydrogencarbonate decomposes (breaks down) to form calcium. During boiling, bicarbonates of calcium and. Water that does not give lather with soap is called hard water. (ii) the permanent hardness of water is removed. When Water Is Boiled The Hardness Removed Is Called.
From www.slideserve.com
PPT HARD WATER AND SOFT WATER PowerPoint Presentation ID2244770 When Water Is Boiled The Hardness Removed Is Called The temporary hardness of water can be removed by the following methods: Boiling will remove cox2 c o x 2, making apparently cacox3 c a. In this case, the hardness in water can be removed by boiling the water. • when the water is boiled,. It reacts with soluble chlorides and. The presence of soluble bicarbonates, of calcium. Water that. When Water Is Boiled The Hardness Removed Is Called.
From www.slideserve.com
PPT Water Hardness PowerPoint Presentation, free download ID2529744 When Water Is Boiled The Hardness Removed Is Called Boiling will evaporate pure water, thus increasing the concentration of minerals including calcium, thereby increasing hardness. • temporary hard water is caused by dissolved calcium hydrogencarbonate or magnesium hydrogencarbonate. (ii) the permanent hardness of water is removed when it is treated with a small quantity of sodium carbonate. Water that can have its hardness removed by boiling it is called. When Water Is Boiled The Hardness Removed Is Called.
From www.slideserve.com
PPT Water Hardness PowerPoint Presentation, free download ID371879 When Water Is Boiled The Hardness Removed Is Called • temporary hard water is caused by dissolved calcium hydrogencarbonate or magnesium hydrogencarbonate. The temporary hardness of water can be removed by the following methods: In this case, the hardness in water can be removed by boiling the water. When we boil water, the soluble salts of mg(hco 3) 2 are converted to mg(oh) 2, which is insoluble, and hence. When Water Is Boiled The Hardness Removed Is Called.
From www.youtube.com
How Temporary Hardness Is Different from Permanent Hardness Water When Water Is Boiled The Hardness Removed Is Called Boiling will remove cox2 c o x 2, making apparently cacox3 c a. In this case, the hardness in water can be removed by boiling the water. The presence of soluble bicarbonates, of calcium. The temporary hardness of water can be removed by the following methods: This method of removing hardness leads. Water that does not give lather with soap. When Water Is Boiled The Hardness Removed Is Called.
From www.slideshare.net
Removing hardness from water When Water Is Boiled The Hardness Removed Is Called During boiling, bicarbonates of calcium and. • when the water is boiled,. It reacts with soluble chlorides and. • temporary hard water is caused by dissolved calcium hydrogencarbonate or magnesium hydrogencarbonate. When temporary hard water is boiled, the soluble calcium hydrogencarbonate decomposes (breaks down) to form calcium. Water that does not give lather with soap is called hard water. In. When Water Is Boiled The Hardness Removed Is Called.
From netsolwater.com
How to remove Hardness from wastewater When Water Is Boiled The Hardness Removed Is Called When temporary hard water is boiled, the soluble calcium hydrogencarbonate decomposes (breaks down) to form calcium. • temporary hard water is caused by dissolved calcium hydrogencarbonate or magnesium hydrogencarbonate. The temporary hardness of water can be removed by the following methods: In this case, the hardness in water can be removed by boiling the water. It reacts with soluble chlorides. When Water Is Boiled The Hardness Removed Is Called.
From www.slideserve.com
PPT Water Hardness PowerPoint Presentation, free download ID371879 When Water Is Boiled The Hardness Removed Is Called In this case, the hardness in water can be removed by boiling the water. • when the water is boiled,. It reacts with soluble chlorides and. When we boil water, the soluble salts of mg(hco 3) 2 are converted to mg(oh) 2, which is insoluble, and hence gets precipitated and is removed. Water that does not give lather with soap. When Water Is Boiled The Hardness Removed Is Called.
From exonzmvvd.blob.core.windows.net
Water Hardness Test Reaction at Anne Howard blog When Water Is Boiled The Hardness Removed Is Called The temporary hardness of water can be removed by the following methods: This method of removing hardness leads. In this case, the hardness in water can be removed by boiling the water. Water that does not give lather with soap is called hard water. • temporary hard water is caused by dissolved calcium hydrogencarbonate or magnesium hydrogencarbonate. (ii) the permanent. When Water Is Boiled The Hardness Removed Is Called.
From www.slideshare.net
water hardness When Water Is Boiled The Hardness Removed Is Called Boiling will remove cox2 c o x 2, making apparently cacox3 c a. It reacts with soluble chlorides and. (ii) the permanent hardness of water is removed when it is treated with a small quantity of sodium carbonate. • temporary hard water is caused by dissolved calcium hydrogencarbonate or magnesium hydrogencarbonate. The presence of soluble bicarbonates, of calcium. This method. When Water Is Boiled The Hardness Removed Is Called.
From www.slideserve.com
PPT HARD WATER AND SOFT WATER PowerPoint Presentation ID2244770 When Water Is Boiled The Hardness Removed Is Called • temporary hard water is caused by dissolved calcium hydrogencarbonate or magnesium hydrogencarbonate. Boiling will remove cox2 c o x 2, making apparently cacox3 c a. Water that does not give lather with soap is called hard water. The presence of soluble bicarbonates, of calcium. • when the water is boiled,. In this case, the hardness in water can be. When Water Is Boiled The Hardness Removed Is Called.
From itechnhealth.com
How Do You Know If You Have Hard Water When Water Is Boiled The Hardness Removed Is Called Water that does not give lather with soap is called hard water. The presence of soluble bicarbonates, of calcium. When we boil water, the soluble salts of mg(hco 3) 2 are converted to mg(oh) 2, which is insoluble, and hence gets precipitated and is removed. • when the water is boiled,. Water that can have its hardness removed by boiling. When Water Is Boiled The Hardness Removed Is Called.
From melscience.com
Water hardness MEL Chemistry When Water Is Boiled The Hardness Removed Is Called Water that does not give lather with soap is called hard water. (ii) the permanent hardness of water is removed when it is treated with a small quantity of sodium carbonate. During boiling, bicarbonates of calcium and. When temporary hard water is boiled, the soluble calcium hydrogencarbonate decomposes (breaks down) to form calcium. • when the water is boiled,. In. When Water Is Boiled The Hardness Removed Is Called.
From www.youtube.com
How to determine hardness of water by EDTA method? (Procedure and When Water Is Boiled The Hardness Removed Is Called It reacts with soluble chlorides and. (ii) the permanent hardness of water is removed when it is treated with a small quantity of sodium carbonate. Boiling will remove cox2 c o x 2, making apparently cacox3 c a. When temporary hard water is boiled, the soluble calcium hydrogencarbonate decomposes (breaks down) to form calcium. This method of removing hardness leads.. When Water Is Boiled The Hardness Removed Is Called.
From mungfali.com
Water Hardness Chart When Water Is Boiled The Hardness Removed Is Called The presence of soluble bicarbonates, of calcium. Water that can have its hardness removed by boiling it is called temporary hard water. Boiling will remove cox2 c o x 2, making apparently cacox3 c a. When temporary hard water is boiled, the soluble calcium hydrogencarbonate decomposes (breaks down) to form calcium. This method of removing hardness leads. During boiling, bicarbonates. When Water Is Boiled The Hardness Removed Is Called.
From www.youtube.com
Softening of Water Method for removing hardness from water How When Water Is Boiled The Hardness Removed Is Called It reacts with soluble chlorides and. When temporary hard water is boiled, the soluble calcium hydrogencarbonate decomposes (breaks down) to form calcium. • temporary hard water is caused by dissolved calcium hydrogencarbonate or magnesium hydrogencarbonate. The presence of soluble bicarbonates, of calcium. Water that does not give lather with soap is called hard water. Boiling will evaporate pure water, thus. When Water Is Boiled The Hardness Removed Is Called.
From netsolwater.com
How does Reverse Osmosis remove the hardness of water When Water Is Boiled The Hardness Removed Is Called Boiling will evaporate pure water, thus increasing the concentration of minerals including calcium, thereby increasing hardness. When we boil water, the soluble salts of mg(hco 3) 2 are converted to mg(oh) 2, which is insoluble, and hence gets precipitated and is removed. • temporary hard water is caused by dissolved calcium hydrogencarbonate or magnesium hydrogencarbonate. During boiling, bicarbonates of calcium. When Water Is Boiled The Hardness Removed Is Called.
From www.slideserve.com
PPT Water Treatment PowerPoint Presentation, free download ID660646 When Water Is Boiled The Hardness Removed Is Called Water that can have its hardness removed by boiling it is called temporary hard water. During boiling, bicarbonates of calcium and. The presence of soluble bicarbonates, of calcium. When we boil water, the soluble salts of mg(hco 3) 2 are converted to mg(oh) 2, which is insoluble, and hence gets precipitated and is removed. Boiling will evaporate pure water, thus. When Water Is Boiled The Hardness Removed Is Called.
From www.slideserve.com
PPT Water hardness & Special treatment PowerPoint Presentation ID When Water Is Boiled The Hardness Removed Is Called The presence of soluble bicarbonates, of calcium. When we boil water, the soluble salts of mg(hco 3) 2 are converted to mg(oh) 2, which is insoluble, and hence gets precipitated and is removed. Water that can have its hardness removed by boiling it is called temporary hard water. During boiling, bicarbonates of calcium and. Water that does not give lather. When Water Is Boiled The Hardness Removed Is Called.
From www.slideserve.com
PPT Hardness of Water PowerPoint Presentation, free download ID2956417 When Water Is Boiled The Hardness Removed Is Called In this case, the hardness in water can be removed by boiling the water. (ii) the permanent hardness of water is removed when it is treated with a small quantity of sodium carbonate. When we boil water, the soluble salts of mg(hco 3) 2 are converted to mg(oh) 2, which is insoluble, and hence gets precipitated and is removed. Water. When Water Is Boiled The Hardness Removed Is Called.
From www.slideserve.com
PPT HARD WATER AND SOFT WATER PowerPoint Presentation, free download When Water Is Boiled The Hardness Removed Is Called This method of removing hardness leads. In this case, the hardness in water can be removed by boiling the water. During boiling, bicarbonates of calcium and. Water that can have its hardness removed by boiling it is called temporary hard water. (ii) the permanent hardness of water is removed when it is treated with a small quantity of sodium carbonate.. When Water Is Boiled The Hardness Removed Is Called.
From www.slideshare.net
water hardness When Water Is Boiled The Hardness Removed Is Called When temporary hard water is boiled, the soluble calcium hydrogencarbonate decomposes (breaks down) to form calcium. The temporary hardness of water can be removed by the following methods: When we boil water, the soluble salts of mg(hco 3) 2 are converted to mg(oh) 2, which is insoluble, and hence gets precipitated and is removed. This method of removing hardness leads.. When Water Is Boiled The Hardness Removed Is Called.
From www.youtube.com
Methods of Removal of Temporary Hardness Water By Boiling and Clark When Water Is Boiled The Hardness Removed Is Called The temporary hardness of water can be removed by the following methods: Boiling will evaporate pure water, thus increasing the concentration of minerals including calcium, thereby increasing hardness. • when the water is boiled,. When temporary hard water is boiled, the soluble calcium hydrogencarbonate decomposes (breaks down) to form calcium. Water that can have its hardness removed by boiling it. When Water Is Boiled The Hardness Removed Is Called.
From www.slideserve.com
PPT Water Treatment PowerPoint Presentation, free download ID660646 When Water Is Boiled The Hardness Removed Is Called In this case, the hardness in water can be removed by boiling the water. This method of removing hardness leads. (ii) the permanent hardness of water is removed when it is treated with a small quantity of sodium carbonate. • temporary hard water is caused by dissolved calcium hydrogencarbonate or magnesium hydrogencarbonate. Boiling will evaporate pure water, thus increasing the. When Water Is Boiled The Hardness Removed Is Called.
From www.slideserve.com
PPT Water hardness & Special treatment PowerPoint Presentation ID When Water Is Boiled The Hardness Removed Is Called This method of removing hardness leads. Water that does not give lather with soap is called hard water. Boiling will evaporate pure water, thus increasing the concentration of minerals including calcium, thereby increasing hardness. Water that can have its hardness removed by boiling it is called temporary hard water. It reacts with soluble chlorides and. (ii) the permanent hardness of. When Water Is Boiled The Hardness Removed Is Called.
From mavink.com
Water Hardness Conversion Chart When Water Is Boiled The Hardness Removed Is Called • when the water is boiled,. It reacts with soluble chlorides and. When we boil water, the soluble salts of mg(hco 3) 2 are converted to mg(oh) 2, which is insoluble, and hence gets precipitated and is removed. The presence of soluble bicarbonates, of calcium. Water that does not give lather with soap is called hard water. During boiling, bicarbonates. When Water Is Boiled The Hardness Removed Is Called.
From www.slideserve.com
PPT Experiment 2 PowerPoint Presentation, free download ID371865 When Water Is Boiled The Hardness Removed Is Called Water that can have its hardness removed by boiling it is called temporary hard water. When temporary hard water is boiled, the soluble calcium hydrogencarbonate decomposes (breaks down) to form calcium. During boiling, bicarbonates of calcium and. • when the water is boiled,. Water that does not give lather with soap is called hard water. When we boil water, the. When Water Is Boiled The Hardness Removed Is Called.
From www.slideserve.com
PPT Water Treatment Basics PowerPoint Presentation, free download When Water Is Boiled The Hardness Removed Is Called Water that does not give lather with soap is called hard water. When temporary hard water is boiled, the soluble calcium hydrogencarbonate decomposes (breaks down) to form calcium. During boiling, bicarbonates of calcium and. This method of removing hardness leads. It reacts with soluble chlorides and. Boiling will remove cox2 c o x 2, making apparently cacox3 c a. •. When Water Is Boiled The Hardness Removed Is Called.
From www.slideshare.net
Hardness of water When Water Is Boiled The Hardness Removed Is Called In this case, the hardness in water can be removed by boiling the water. When we boil water, the soluble salts of mg(hco 3) 2 are converted to mg(oh) 2, which is insoluble, and hence gets precipitated and is removed. Boiling will evaporate pure water, thus increasing the concentration of minerals including calcium, thereby increasing hardness. Boiling will remove cox2. When Water Is Boiled The Hardness Removed Is Called.
From graingerrevise.blogspot.com
Chemistry Revision C6f Hardness of Water When Water Is Boiled The Hardness Removed Is Called • temporary hard water is caused by dissolved calcium hydrogencarbonate or magnesium hydrogencarbonate. The presence of soluble bicarbonates, of calcium. This method of removing hardness leads. Boiling will remove cox2 c o x 2, making apparently cacox3 c a. (ii) the permanent hardness of water is removed when it is treated with a small quantity of sodium carbonate. The temporary. When Water Is Boiled The Hardness Removed Is Called.
From www.slideserve.com
PPT IV. Water Chemistry PowerPoint Presentation, free download ID When Water Is Boiled The Hardness Removed Is Called When temporary hard water is boiled, the soluble calcium hydrogencarbonate decomposes (breaks down) to form calcium. Water that can have its hardness removed by boiling it is called temporary hard water. Boiling will evaporate pure water, thus increasing the concentration of minerals including calcium, thereby increasing hardness. In this case, the hardness in water can be removed by boiling the. When Water Is Boiled The Hardness Removed Is Called.
From www.westernvawater.org
Water Hardness Western Virginia Water Authority When Water Is Boiled The Hardness Removed Is Called Boiling will evaporate pure water, thus increasing the concentration of minerals including calcium, thereby increasing hardness. The presence of soluble bicarbonates, of calcium. When temporary hard water is boiled, the soluble calcium hydrogencarbonate decomposes (breaks down) to form calcium. • temporary hard water is caused by dissolved calcium hydrogencarbonate or magnesium hydrogencarbonate. Water that does not give lather with soap. When Water Is Boiled The Hardness Removed Is Called.