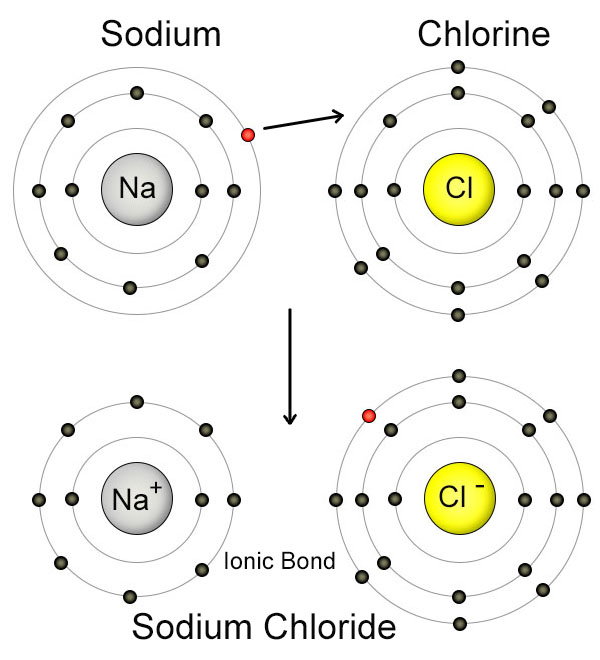
Sodium Chloride Or Table Salt Is An Example Of A Bond . The classic example is table salt or sodium chloride. In chemistry, a salt is an electrically neutral chemical compound consisting of cations and anions connected by an ionic bond. Ionic bonding in sodium chloride. For example, when sodium reacts with chlorine, electrons transfer from sodium atoms to chlorine atoms. The ions in sodium chloride form an ionic bond, which is the electrostatic attraction between the positively charged sodium ions and the negatively charged chloride. The diagrams show two ways of representing this electron transfer. An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion (na +) and.
from cabinet.matttroy.net
For example, when sodium reacts with chlorine, electrons transfer from sodium atoms to chlorine atoms. In chemistry, a salt is an electrically neutral chemical compound consisting of cations and anions connected by an ionic bond. The ions in sodium chloride form an ionic bond, which is the electrostatic attraction between the positively charged sodium ions and the negatively charged chloride. The classic example is table salt or sodium chloride. An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion (na +) and. The diagrams show two ways of representing this electron transfer. Ionic bonding in sodium chloride.
Is Table Salt A Molecule Or Compound Matttroy
Sodium Chloride Or Table Salt Is An Example Of A Bond In chemistry, a salt is an electrically neutral chemical compound consisting of cations and anions connected by an ionic bond. The ions in sodium chloride form an ionic bond, which is the electrostatic attraction between the positively charged sodium ions and the negatively charged chloride. For example, when sodium reacts with chlorine, electrons transfer from sodium atoms to chlorine atoms. In chemistry, a salt is an electrically neutral chemical compound consisting of cations and anions connected by an ionic bond. The diagrams show two ways of representing this electron transfer. Ionic bonding in sodium chloride. The classic example is table salt or sodium chloride. An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion (na +) and.
From stock.adobe.com
Structure of sodium chloride (salt).NaCl model.Vector illustration Sodium Chloride Or Table Salt Is An Example Of A Bond The classic example is table salt or sodium chloride. Ionic bonding in sodium chloride. The ions in sodium chloride form an ionic bond, which is the electrostatic attraction between the positively charged sodium ions and the negatively charged chloride. In chemistry, a salt is an electrically neutral chemical compound consisting of cations and anions connected by an ionic bond. An. Sodium Chloride Or Table Salt Is An Example Of A Bond.
From www.pikpng.com
Download Png Royalty Free Drawing Atoms Sodium Covalent Bonding Of Sodium Chloride Or Table Salt Is An Example Of A Bond The diagrams show two ways of representing this electron transfer. In chemistry, a salt is an electrically neutral chemical compound consisting of cations and anions connected by an ionic bond. The ions in sodium chloride form an ionic bond, which is the electrostatic attraction between the positively charged sodium ions and the negatively charged chloride. The classic example is table. Sodium Chloride Or Table Salt Is An Example Of A Bond.
From slideplayer.com
To get Chromebook Log into Classroom ppt download Sodium Chloride Or Table Salt Is An Example Of A Bond The ions in sodium chloride form an ionic bond, which is the electrostatic attraction between the positively charged sodium ions and the negatively charged chloride. The classic example is table salt or sodium chloride. The diagrams show two ways of representing this electron transfer. For example, when sodium reacts with chlorine, electrons transfer from sodium atoms to chlorine atoms. An. Sodium Chloride Or Table Salt Is An Example Of A Bond.
From www.shutterstock.com
Ionic Bond Sodium Chloride Table Salt Stock Vector (Royalty Free Sodium Chloride Or Table Salt Is An Example Of A Bond In chemistry, a salt is an electrically neutral chemical compound consisting of cations and anions connected by an ionic bond. An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion (na +) and. The diagrams show two ways of representing this electron transfer. For example,. Sodium Chloride Or Table Salt Is An Example Of A Bond.
From www.shutterstock.com
Sodium Chloride Known Salt Table Salt Stock Photo 2176288651 Shutterstock Sodium Chloride Or Table Salt Is An Example Of A Bond The classic example is table salt or sodium chloride. For example, when sodium reacts with chlorine, electrons transfer from sodium atoms to chlorine atoms. In chemistry, a salt is an electrically neutral chemical compound consisting of cations and anions connected by an ionic bond. An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl). Sodium Chloride Or Table Salt Is An Example Of A Bond.
From stock.adobe.com
Crystal structure of Sodium chloride and diatomic molecule of salt Sodium Chloride Or Table Salt Is An Example Of A Bond For example, when sodium reacts with chlorine, electrons transfer from sodium atoms to chlorine atoms. The diagrams show two ways of representing this electron transfer. The classic example is table salt or sodium chloride. Ionic bonding in sodium chloride. The ions in sodium chloride form an ionic bond, which is the electrostatic attraction between the positively charged sodium ions and. Sodium Chloride Or Table Salt Is An Example Of A Bond.
From sphweb.bumc.bu.edu
Chemical Elements Atoms Sodium Chloride Or Table Salt Is An Example Of A Bond The ions in sodium chloride form an ionic bond, which is the electrostatic attraction between the positively charged sodium ions and the negatively charged chloride. Ionic bonding in sodium chloride. The diagrams show two ways of representing this electron transfer. In chemistry, a salt is an electrically neutral chemical compound consisting of cations and anions connected by an ionic bond.. Sodium Chloride Or Table Salt Is An Example Of A Bond.
From www.slideserve.com
PPT Making Molecules PowerPoint Presentation, free download ID6226902 Sodium Chloride Or Table Salt Is An Example Of A Bond The classic example is table salt or sodium chloride. The diagrams show two ways of representing this electron transfer. Ionic bonding in sodium chloride. The ions in sodium chloride form an ionic bond, which is the electrostatic attraction between the positively charged sodium ions and the negatively charged chloride. In chemistry, a salt is an electrically neutral chemical compound consisting. Sodium Chloride Or Table Salt Is An Example Of A Bond.
From eduinput.com
30 Examples Of Common Chemical Compounds with Chemical Formula Sodium Chloride Or Table Salt Is An Example Of A Bond The diagrams show two ways of representing this electron transfer. The classic example is table salt or sodium chloride. For example, when sodium reacts with chlorine, electrons transfer from sodium atoms to chlorine atoms. In chemistry, a salt is an electrically neutral chemical compound consisting of cations and anions connected by an ionic bond. The ions in sodium chloride form. Sodium Chloride Or Table Salt Is An Example Of A Bond.
From www.alamy.com
Sodium Chloride ionic bond formation. NaCl structure. Sodium and Sodium Chloride Or Table Salt Is An Example Of A Bond The classic example is table salt or sodium chloride. The diagrams show two ways of representing this electron transfer. The ions in sodium chloride form an ionic bond, which is the electrostatic attraction between the positively charged sodium ions and the negatively charged chloride. In chemistry, a salt is an electrically neutral chemical compound consisting of cations and anions connected. Sodium Chloride Or Table Salt Is An Example Of A Bond.
From avopix.com
ionic bond sodium chloride, or table salt. Royalty Free Stock Vector Sodium Chloride Or Table Salt Is An Example Of A Bond The diagrams show two ways of representing this electron transfer. The classic example is table salt or sodium chloride. The ions in sodium chloride form an ionic bond, which is the electrostatic attraction between the positively charged sodium ions and the negatively charged chloride. For example, when sodium reacts with chlorine, electrons transfer from sodium atoms to chlorine atoms. An. Sodium Chloride Or Table Salt Is An Example Of A Bond.
From www.alamy.com
Sodium chloride (table salt), chemical structure. Blue skeletal formula Sodium Chloride Or Table Salt Is An Example Of A Bond The ions in sodium chloride form an ionic bond, which is the electrostatic attraction between the positively charged sodium ions and the negatively charged chloride. For example, when sodium reacts with chlorine, electrons transfer from sodium atoms to chlorine atoms. In chemistry, a salt is an electrically neutral chemical compound consisting of cations and anions connected by an ionic bond.. Sodium Chloride Or Table Salt Is An Example Of A Bond.
From mavink.com
Sodium Chloride Bonding Diagram Sodium Chloride Or Table Salt Is An Example Of A Bond The diagrams show two ways of representing this electron transfer. The ions in sodium chloride form an ionic bond, which is the electrostatic attraction between the positively charged sodium ions and the negatively charged chloride. An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion. Sodium Chloride Or Table Salt Is An Example Of A Bond.
From stock.adobe.com
Sodium chloride (table salt), chemical structure. Skeletal formula Sodium Chloride Or Table Salt Is An Example Of A Bond The ions in sodium chloride form an ionic bond, which is the electrostatic attraction between the positively charged sodium ions and the negatively charged chloride. An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion (na +) and. The classic example is table salt or. Sodium Chloride Or Table Salt Is An Example Of A Bond.
From abbeyl-dane.blogspot.com
Nacl / Sodium Chloride Nacl Table Salt Crystal Structure Atoms Are Sodium Chloride Or Table Salt Is An Example Of A Bond The ions in sodium chloride form an ionic bond, which is the electrostatic attraction between the positively charged sodium ions and the negatively charged chloride. In chemistry, a salt is an electrically neutral chemical compound consisting of cations and anions connected by an ionic bond. The diagrams show two ways of representing this electron transfer. For example, when sodium reacts. Sodium Chloride Or Table Salt Is An Example Of A Bond.
From www.animalia-life.club
Table Salt Chemical Structure Sodium Chloride Or Table Salt Is An Example Of A Bond Ionic bonding in sodium chloride. The diagrams show two ways of representing this electron transfer. An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion (na +) and. The ions in sodium chloride form an ionic bond, which is the electrostatic attraction between the positively. Sodium Chloride Or Table Salt Is An Example Of A Bond.
From www.istockphoto.com
Vector Ballandstick Model Of Chemical Substance Icon Of Sodium Chloride Sodium Chloride Or Table Salt Is An Example Of A Bond The classic example is table salt or sodium chloride. For example, when sodium reacts with chlorine, electrons transfer from sodium atoms to chlorine atoms. In chemistry, a salt is an electrically neutral chemical compound consisting of cations and anions connected by an ionic bond. The ions in sodium chloride form an ionic bond, which is the electrostatic attraction between the. Sodium Chloride Or Table Salt Is An Example Of A Bond.
From byjus.com
Sodium Chloride Preparation, Properties, Structure & Uses Byju's Sodium Chloride Or Table Salt Is An Example Of A Bond An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion (na +) and. For example, when sodium reacts with chlorine, electrons transfer from sodium atoms to chlorine atoms. The ions in sodium chloride form an ionic bond, which is the electrostatic attraction between the positively. Sodium Chloride Or Table Salt Is An Example Of A Bond.
From avopix.com
ionic bond sodium chloride, or table salt. Royalty Free Stock Vector Sodium Chloride Or Table Salt Is An Example Of A Bond The classic example is table salt or sodium chloride. The diagrams show two ways of representing this electron transfer. For example, when sodium reacts with chlorine, electrons transfer from sodium atoms to chlorine atoms. An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion (na. Sodium Chloride Or Table Salt Is An Example Of A Bond.
From www.thoughtco.com
Chemical Composition of Table Salt Sodium Chloride Or Table Salt Is An Example Of A Bond An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion (na +) and. The ions in sodium chloride form an ionic bond, which is the electrostatic attraction between the positively charged sodium ions and the negatively charged chloride. In chemistry, a salt is an electrically. Sodium Chloride Or Table Salt Is An Example Of A Bond.
From www.thoughtco.com
Table Salt Molecular Formula Sodium Chloride Sodium Chloride Or Table Salt Is An Example Of A Bond The diagrams show two ways of representing this electron transfer. The ions in sodium chloride form an ionic bond, which is the electrostatic attraction between the positively charged sodium ions and the negatively charged chloride. In chemistry, a salt is an electrically neutral chemical compound consisting of cations and anions connected by an ionic bond. Ionic bonding in sodium chloride.. Sodium Chloride Or Table Salt Is An Example Of A Bond.
From www.shalom-education.com
Properties of Ionic Compounds GCSE Chemistry Revision Sodium Chloride Or Table Salt Is An Example Of A Bond The classic example is table salt or sodium chloride. The diagrams show two ways of representing this electron transfer. Ionic bonding in sodium chloride. For example, when sodium reacts with chlorine, electrons transfer from sodium atoms to chlorine atoms. In chemistry, a salt is an electrically neutral chemical compound consisting of cations and anions connected by an ionic bond. An. Sodium Chloride Or Table Salt Is An Example Of A Bond.
From www.britannica.com
ionic bond Definition, Properties, Examples, & Facts Britannica Sodium Chloride Or Table Salt Is An Example Of A Bond Ionic bonding in sodium chloride. In chemistry, a salt is an electrically neutral chemical compound consisting of cations and anions connected by an ionic bond. The diagrams show two ways of representing this electron transfer. For example, when sodium reacts with chlorine, electrons transfer from sodium atoms to chlorine atoms. The classic example is table salt or sodium chloride. An. Sodium Chloride Or Table Salt Is An Example Of A Bond.
From www.alamy.com
Sodium chloride, NaCl crystal structure with sodium in gray and Sodium Chloride Or Table Salt Is An Example Of A Bond For example, when sodium reacts with chlorine, electrons transfer from sodium atoms to chlorine atoms. The classic example is table salt or sodium chloride. The ions in sodium chloride form an ionic bond, which is the electrostatic attraction between the positively charged sodium ions and the negatively charged chloride. Ionic bonding in sodium chloride. In chemistry, a salt is an. Sodium Chloride Or Table Salt Is An Example Of A Bond.
From www.freepik.com
Premium Vector Sodium chloride table salt rock salt halite chemical Sodium Chloride Or Table Salt Is An Example Of A Bond The ions in sodium chloride form an ionic bond, which is the electrostatic attraction between the positively charged sodium ions and the negatively charged chloride. An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion (na +) and. The classic example is table salt or. Sodium Chloride Or Table Salt Is An Example Of A Bond.
From www.dreamstime.com
Sodium Chloride or Rock Salt, Halite, Table Salt, Chemical Structure Sodium Chloride Or Table Salt Is An Example Of A Bond In chemistry, a salt is an electrically neutral chemical compound consisting of cations and anions connected by an ionic bond. The classic example is table salt or sodium chloride. The diagrams show two ways of representing this electron transfer. Ionic bonding in sodium chloride. For example, when sodium reacts with chlorine, electrons transfer from sodium atoms to chlorine atoms. The. Sodium Chloride Or Table Salt Is An Example Of A Bond.
From cabinet.matttroy.net
Is Table Salt A Molecule Or Compound Matttroy Sodium Chloride Or Table Salt Is An Example Of A Bond The ions in sodium chloride form an ionic bond, which is the electrostatic attraction between the positively charged sodium ions and the negatively charged chloride. An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion (na +) and. For example, when sodium reacts with chlorine,. Sodium Chloride Or Table Salt Is An Example Of A Bond.
From sciencenotes.org
What Is a Salt in Chemistry? Definition and Examples Sodium Chloride Or Table Salt Is An Example Of A Bond Ionic bonding in sodium chloride. In chemistry, a salt is an electrically neutral chemical compound consisting of cations and anions connected by an ionic bond. The classic example is table salt or sodium chloride. For example, when sodium reacts with chlorine, electrons transfer from sodium atoms to chlorine atoms. An atom of sodium (na) donates one of its electrons to. Sodium Chloride Or Table Salt Is An Example Of A Bond.
From www.youtube.com
Structure of NaCl (Sodium chloride) YouTube Sodium Chloride Or Table Salt Is An Example Of A Bond Ionic bonding in sodium chloride. For example, when sodium reacts with chlorine, electrons transfer from sodium atoms to chlorine atoms. In chemistry, a salt is an electrically neutral chemical compound consisting of cations and anions connected by an ionic bond. The diagrams show two ways of representing this electron transfer. The classic example is table salt or sodium chloride. An. Sodium Chloride Or Table Salt Is An Example Of A Bond.
From www.slideserve.com
PPT Ionic Bonding PowerPoint Presentation, free download ID5679234 Sodium Chloride Or Table Salt Is An Example Of A Bond For example, when sodium reacts with chlorine, electrons transfer from sodium atoms to chlorine atoms. An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion (na +) and. The diagrams show two ways of representing this electron transfer. The ions in sodium chloride form an. Sodium Chloride Or Table Salt Is An Example Of A Bond.
From www.alamy.com
Sodium chloride molecule, salt chemical structures vector Stock Vector Sodium Chloride Or Table Salt Is An Example Of A Bond For example, when sodium reacts with chlorine, electrons transfer from sodium atoms to chlorine atoms. The diagrams show two ways of representing this electron transfer. Ionic bonding in sodium chloride. An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion (na +) and. The ions. Sodium Chloride Or Table Salt Is An Example Of A Bond.
From www.shutterstock.com
Sodium Chloride Table Salt Chemical Structure Stock Vector (Royalty Sodium Chloride Or Table Salt Is An Example Of A Bond The diagrams show two ways of representing this electron transfer. In chemistry, a salt is an electrically neutral chemical compound consisting of cations and anions connected by an ionic bond. The ions in sodium chloride form an ionic bond, which is the electrostatic attraction between the positively charged sodium ions and the negatively charged chloride. For example, when sodium reacts. Sodium Chloride Or Table Salt Is An Example Of A Bond.
From chem.libretexts.org
Ionic Solids Chemistry LibreTexts Sodium Chloride Or Table Salt Is An Example Of A Bond In chemistry, a salt is an electrically neutral chemical compound consisting of cations and anions connected by an ionic bond. For example, when sodium reacts with chlorine, electrons transfer from sodium atoms to chlorine atoms. The ions in sodium chloride form an ionic bond, which is the electrostatic attraction between the positively charged sodium ions and the negatively charged chloride.. Sodium Chloride Or Table Salt Is An Example Of A Bond.
From studiousguy.com
Sodium (Na) Properties & Uses StudiousGuy Sodium Chloride Or Table Salt Is An Example Of A Bond An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion (na +) and. Ionic bonding in sodium chloride. In chemistry, a salt is an electrically neutral chemical compound consisting of cations and anions connected by an ionic bond. The diagrams show two ways of representing. Sodium Chloride Or Table Salt Is An Example Of A Bond.
From www.dreamstime.com
Diagram To Show Ionic Bonding in Sodium Chloride Stock Illustration Sodium Chloride Or Table Salt Is An Example Of A Bond For example, when sodium reacts with chlorine, electrons transfer from sodium atoms to chlorine atoms. An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion (na +) and. The classic example is table salt or sodium chloride. The diagrams show two ways of representing this. Sodium Chloride Or Table Salt Is An Example Of A Bond.