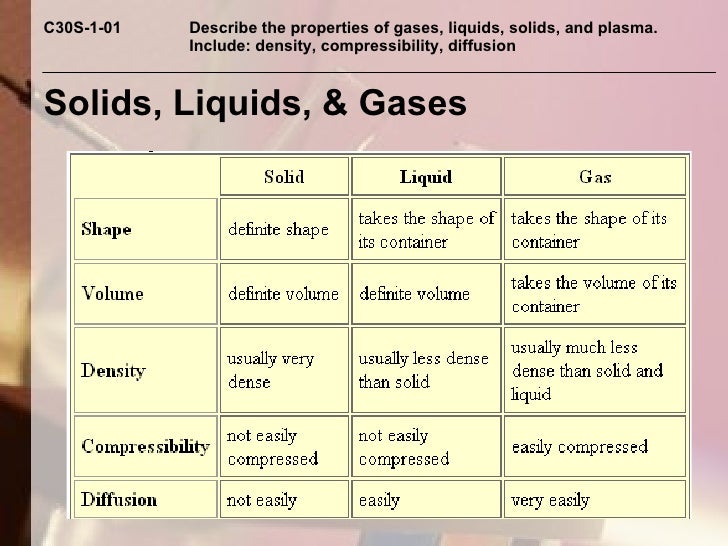
Solid Liquid And Gas Compressibility . And why they adopt the shape (but not the volume) of their containers. The thermal expansion of liquids; Gases are highly compressible compared to solids and liquids due to the larger. In a solid state of matter: In a solid like this brick, the particles are regularly arranged touching their neighbours and move only by vibrating. Comparison of the compressibility of solids, liquids, and gases: This explains why solids have a fixed shape. Why does a gas have no definite shape and no definite. Compressibility is the measure of reducing the volume of a substance when pressure is applied to it. How are shape and volume used to classify solids, liquids, and gases? This model explains the higher density, greater order, and lower compressibility of liquids versus gases; There are three different states of matter: Unlike solids and liquids, gases are highly compressible ; Gases are very compressible, so when subjected to high pressures, their volumes decrease significantly (think boyle’s law!) solids and.
from www.slideshare.net
This model explains the higher density, greater order, and lower compressibility of liquids versus gases; Gases are very compressible, so when subjected to high pressures, their volumes decrease significantly (think boyle’s law!) solids and. How are shape and volume used to classify solids, liquids, and gases? Why does a gas have no definite shape and no definite. Unlike solids and liquids, gases are highly compressible ; Comparison of the compressibility of solids, liquids, and gases: In a solid state of matter: This explains why solids have a fixed shape. There are three different states of matter: In a solid like this brick, the particles are regularly arranged touching their neighbours and move only by vibrating.
Unit 1 Notes
Solid Liquid And Gas Compressibility Gases are highly compressible compared to solids and liquids due to the larger. Why does a gas have no definite shape and no definite. The thermal expansion of liquids; Unlike solids and liquids, gases are highly compressible ; This model explains the higher density, greater order, and lower compressibility of liquids versus gases; How are shape and volume used to classify solids, liquids, and gases? And why they adopt the shape (but not the volume) of their containers. In a solid like this brick, the particles are regularly arranged touching their neighbours and move only by vibrating. In a solid state of matter: Compressibility is the measure of reducing the volume of a substance when pressure is applied to it. Comparison of the compressibility of solids, liquids, and gases: There are three different states of matter: Gases are very compressible, so when subjected to high pressures, their volumes decrease significantly (think boyle’s law!) solids and. Gases are highly compressible compared to solids and liquids due to the larger. This explains why solids have a fixed shape.
From djwvribteco.blob.core.windows.net
Diffusion In Solids Liquids Gases Jost at Mary Foster blog Solid Liquid And Gas Compressibility And why they adopt the shape (but not the volume) of their containers. Gases are very compressible, so when subjected to high pressures, their volumes decrease significantly (think boyle’s law!) solids and. There are three different states of matter: Comparison of the compressibility of solids, liquids, and gases: This model explains the higher density, greater order, and lower compressibility of. Solid Liquid And Gas Compressibility.
From exyhlknsu.blob.core.windows.net
Solids Liquids And Gases Ks2 Powerpoint at Bernice blog Solid Liquid And Gas Compressibility And why they adopt the shape (but not the volume) of their containers. Gases are highly compressible compared to solids and liquids due to the larger. There are three different states of matter: In a solid like this brick, the particles are regularly arranged touching their neighbours and move only by vibrating. How are shape and volume used to classify. Solid Liquid And Gas Compressibility.
From www.gasrebate.net
Properties Of Solids Liquids Gases Compared Teachoo Science Solid Liquid And Gas Compressibility Why does a gas have no definite shape and no definite. And why they adopt the shape (but not the volume) of their containers. Unlike solids and liquids, gases are highly compressible ; Compressibility is the measure of reducing the volume of a substance when pressure is applied to it. This model explains the higher density, greater order, and lower. Solid Liquid And Gas Compressibility.
From studylib.net
Solids liquids gases plasma Solid Liquid And Gas Compressibility Unlike solids and liquids, gases are highly compressible ; The thermal expansion of liquids; Gases are very compressible, so when subjected to high pressures, their volumes decrease significantly (think boyle’s law!) solids and. How are shape and volume used to classify solids, liquids, and gases? This explains why solids have a fixed shape. There are three different states of matter:. Solid Liquid And Gas Compressibility.
From www.slideserve.com
PPT SOLIDS LIQUIDS GASES PowerPoint Presentation, free download ID Solid Liquid And Gas Compressibility In a solid like this brick, the particles are regularly arranged touching their neighbours and move only by vibrating. This model explains the higher density, greater order, and lower compressibility of liquids versus gases; There are three different states of matter: Why does a gas have no definite shape and no definite. The thermal expansion of liquids; How are shape. Solid Liquid And Gas Compressibility.
From dxojwesjc.blob.core.windows.net
Lesson Plan Properties Of Solids Liquids And Gases at Albert Mitchell blog Solid Liquid And Gas Compressibility The thermal expansion of liquids; Unlike solids and liquids, gases are highly compressible ; Gases are very compressible, so when subjected to high pressures, their volumes decrease significantly (think boyle’s law!) solids and. In a solid like this brick, the particles are regularly arranged touching their neighbours and move only by vibrating. Why does a gas have no definite shape. Solid Liquid And Gas Compressibility.
From studymaskirnex5.z21.web.core.windows.net
Science Solid Solid Liquid And Gas Compressibility Unlike solids and liquids, gases are highly compressible ; Why does a gas have no definite shape and no definite. In a solid state of matter: In a solid like this brick, the particles are regularly arranged touching their neighbours and move only by vibrating. There are three different states of matter: Gases are very compressible, so when subjected to. Solid Liquid And Gas Compressibility.
From www.yaclass.in
Compressibility of solids, liquids and gases — lesson. Science State Solid Liquid And Gas Compressibility There are three different states of matter: In a solid state of matter: Comparison of the compressibility of solids, liquids, and gases: Why does a gas have no definite shape and no definite. This explains why solids have a fixed shape. This model explains the higher density, greater order, and lower compressibility of liquids versus gases; The thermal expansion of. Solid Liquid And Gas Compressibility.
From exokrxpvb.blob.core.windows.net
Solid Liquid at Sherry Woodberry blog Solid Liquid And Gas Compressibility In a solid like this brick, the particles are regularly arranged touching their neighbours and move only by vibrating. Comparison of the compressibility of solids, liquids, and gases: And why they adopt the shape (but not the volume) of their containers. Compressibility is the measure of reducing the volume of a substance when pressure is applied to it. The thermal. Solid Liquid And Gas Compressibility.
From igcsechemistryrevision.weebly.com
1.1 Understand the arrangement, movement and energy of particles in Solid Liquid And Gas Compressibility Gases are very compressible, so when subjected to high pressures, their volumes decrease significantly (think boyle’s law!) solids and. The thermal expansion of liquids; Gases are highly compressible compared to solids and liquids due to the larger. Comparison of the compressibility of solids, liquids, and gases: How are shape and volume used to classify solids, liquids, and gases? In a. Solid Liquid And Gas Compressibility.
From www.slideshare.net
Compressibility of solids , liquids and gases Solid Liquid And Gas Compressibility This model explains the higher density, greater order, and lower compressibility of liquids versus gases; In a solid state of matter: Gases are very compressible, so when subjected to high pressures, their volumes decrease significantly (think boyle’s law!) solids and. And why they adopt the shape (but not the volume) of their containers. In a solid like this brick, the. Solid Liquid And Gas Compressibility.
From www.slideshare.net
Unit 1 Notes Solid Liquid And Gas Compressibility This explains why solids have a fixed shape. Comparison of the compressibility of solids, liquids, and gases: Gases are very compressible, so when subjected to high pressures, their volumes decrease significantly (think boyle’s law!) solids and. How are shape and volume used to classify solids, liquids, and gases? In a solid state of matter: There are three different states of. Solid Liquid And Gas Compressibility.
From giokinxez.blob.core.windows.net
Copper Element Solid Liquid Or Gas at Archie Adamski blog Solid Liquid And Gas Compressibility In a solid like this brick, the particles are regularly arranged touching their neighbours and move only by vibrating. And why they adopt the shape (but not the volume) of their containers. How are shape and volume used to classify solids, liquids, and gases? This explains why solids have a fixed shape. There are three different states of matter: The. Solid Liquid And Gas Compressibility.
From www.pinterest.com
Difference between Solid, Liquid and Gas in Tabular form Solid liquid Solid Liquid And Gas Compressibility In a solid like this brick, the particles are regularly arranged touching their neighbours and move only by vibrating. Compressibility is the measure of reducing the volume of a substance when pressure is applied to it. This explains why solids have a fixed shape. How are shape and volume used to classify solids, liquids, and gases? Why does a gas. Solid Liquid And Gas Compressibility.
From brainly.in
difference between solid , liquid and gas on the basis of mass Solid Liquid And Gas Compressibility In a solid like this brick, the particles are regularly arranged touching their neighbours and move only by vibrating. Comparison of the compressibility of solids, liquids, and gases: The thermal expansion of liquids; Gases are highly compressible compared to solids and liquids due to the larger. Unlike solids and liquids, gases are highly compressible ; There are three different states. Solid Liquid And Gas Compressibility.
From www.pinterest.com
States of Matter solids, liquids and gases Chemistry for All The Solid Liquid And Gas Compressibility Gases are highly compressible compared to solids and liquids due to the larger. In a solid like this brick, the particles are regularly arranged touching their neighbours and move only by vibrating. This model explains the higher density, greater order, and lower compressibility of liquids versus gases; There are three different states of matter: How are shape and volume used. Solid Liquid And Gas Compressibility.
From www.youtube.com
States of matter 🚗💧☁️ Solid, Liquid & Gas Learn with examples YouTube Solid Liquid And Gas Compressibility Gases are very compressible, so when subjected to high pressures, their volumes decrease significantly (think boyle’s law!) solids and. In a solid state of matter: Why does a gas have no definite shape and no definite. Compressibility is the measure of reducing the volume of a substance when pressure is applied to it. This explains why solids have a fixed. Solid Liquid And Gas Compressibility.
From gabybonilla10.blogspot.com
science class activity voabulary solids liquids and gases Solid Liquid And Gas Compressibility This model explains the higher density, greater order, and lower compressibility of liquids versus gases; Gases are highly compressible compared to solids and liquids due to the larger. Unlike solids and liquids, gases are highly compressible ; And why they adopt the shape (but not the volume) of their containers. Comparison of the compressibility of solids, liquids, and gases: How. Solid Liquid And Gas Compressibility.
From psiberg.com
Properties of Solid, Liquid, Gases A Comparison Solid Liquid And Gas Compressibility Unlike solids and liquids, gases are highly compressible ; This explains why solids have a fixed shape. The thermal expansion of liquids; And why they adopt the shape (but not the volume) of their containers. Gases are very compressible, so when subjected to high pressures, their volumes decrease significantly (think boyle’s law!) solids and. In a solid like this brick,. Solid Liquid And Gas Compressibility.
From general.chemistrysteps.com
States of Matter Solid, Liquid, Gas, and Plasma Chemistry Steps Solid Liquid And Gas Compressibility Gases are very compressible, so when subjected to high pressures, their volumes decrease significantly (think boyle’s law!) solids and. In a solid like this brick, the particles are regularly arranged touching their neighbours and move only by vibrating. The thermal expansion of liquids; This explains why solids have a fixed shape. Comparison of the compressibility of solids, liquids, and gases:. Solid Liquid And Gas Compressibility.
From jordynfersmcguire.blogspot.com
Comparison Between Solid Liquid and Gas Solid Liquid And Gas Compressibility In a solid state of matter: Why does a gas have no definite shape and no definite. Gases are very compressible, so when subjected to high pressures, their volumes decrease significantly (think boyle’s law!) solids and. The thermal expansion of liquids; There are three different states of matter: This model explains the higher density, greater order, and lower compressibility of. Solid Liquid And Gas Compressibility.
From middleschoolscience.com
Solid, Liquid, & Gas Triple Venn Diagram Activity Middle School Solid Liquid And Gas Compressibility The thermal expansion of liquids; Gases are very compressible, so when subjected to high pressures, their volumes decrease significantly (think boyle’s law!) solids and. In a solid like this brick, the particles are regularly arranged touching their neighbours and move only by vibrating. Comparison of the compressibility of solids, liquids, and gases: This model explains the higher density, greater order,. Solid Liquid And Gas Compressibility.
From www.yaclass.in
Compressibility of solids, liquids and gases — lesson. Science State Solid Liquid And Gas Compressibility This explains why solids have a fixed shape. The thermal expansion of liquids; And why they adopt the shape (but not the volume) of their containers. In a solid like this brick, the particles are regularly arranged touching their neighbours and move only by vibrating. There are three different states of matter: Why does a gas have no definite shape. Solid Liquid And Gas Compressibility.