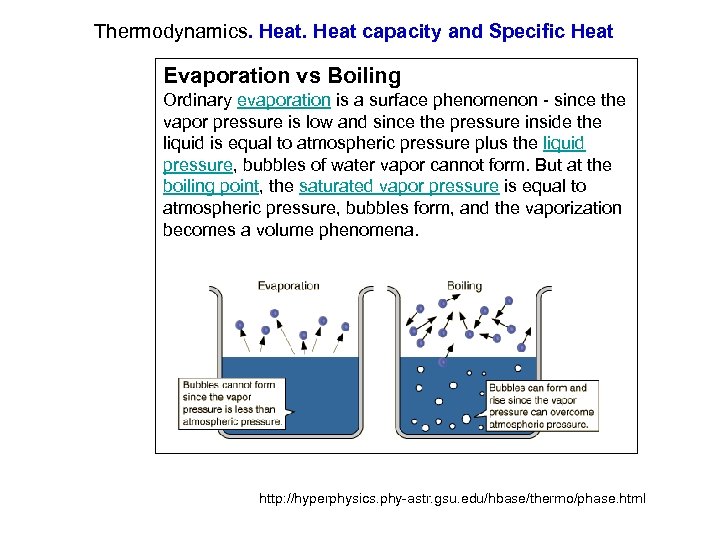
Evaporation Heat Capacity Water . Special properties of water are its high heat capacity and heat of vaporization, its ability to dissolve polar molecules, its cohesive and adhesive properties, and its dissociation into ions. I was wondering how to calculate the mass of water that evaporates if i were to expose the water to some amount of heat given by q. 53 rows figures and tables showing how the properties of water changes along the boiling/condensation curve (vapor pressure,. At room temperature the specific heat of liquid water is 4.18 j/ (g ⋅ ⋅ k) while the ethalpy of evaporation is 44.0 kj/mol. Thermal properties of water at different temperatures like density, freezing temperature, boiling temperature, latent heat of melting,.
from present5.com
Special properties of water are its high heat capacity and heat of vaporization, its ability to dissolve polar molecules, its cohesive and adhesive properties, and its dissociation into ions. I was wondering how to calculate the mass of water that evaporates if i were to expose the water to some amount of heat given by q. 53 rows figures and tables showing how the properties of water changes along the boiling/condensation curve (vapor pressure,. At room temperature the specific heat of liquid water is 4.18 j/ (g ⋅ ⋅ k) while the ethalpy of evaporation is 44.0 kj/mol. Thermal properties of water at different temperatures like density, freezing temperature, boiling temperature, latent heat of melting,.
Thermodynamics I Te mperature Thermal Equilibrium and
Evaporation Heat Capacity Water At room temperature the specific heat of liquid water is 4.18 j/ (g ⋅ ⋅ k) while the ethalpy of evaporation is 44.0 kj/mol. Thermal properties of water at different temperatures like density, freezing temperature, boiling temperature, latent heat of melting,. Special properties of water are its high heat capacity and heat of vaporization, its ability to dissolve polar molecules, its cohesive and adhesive properties, and its dissociation into ions. I was wondering how to calculate the mass of water that evaporates if i were to expose the water to some amount of heat given by q. At room temperature the specific heat of liquid water is 4.18 j/ (g ⋅ ⋅ k) while the ethalpy of evaporation is 44.0 kj/mol. 53 rows figures and tables showing how the properties of water changes along the boiling/condensation curve (vapor pressure,.
From www.expii.com
Heat Capacity of Water — Overview & Importance Expii Evaporation Heat Capacity Water I was wondering how to calculate the mass of water that evaporates if i were to expose the water to some amount of heat given by q. Special properties of water are its high heat capacity and heat of vaporization, its ability to dissolve polar molecules, its cohesive and adhesive properties, and its dissociation into ions. Thermal properties of water. Evaporation Heat Capacity Water.
From www.teachoo.com
Latent Heat of Vaporization and Fusion Definition Teachoo Evaporation Heat Capacity Water I was wondering how to calculate the mass of water that evaporates if i were to expose the water to some amount of heat given by q. At room temperature the specific heat of liquid water is 4.18 j/ (g ⋅ ⋅ k) while the ethalpy of evaporation is 44.0 kj/mol. 53 rows figures and tables showing how the properties. Evaporation Heat Capacity Water.
From www.researchgate.net
The influence of effect numbers on the evaporation capacity and heat Evaporation Heat Capacity Water At room temperature the specific heat of liquid water is 4.18 j/ (g ⋅ ⋅ k) while the ethalpy of evaporation is 44.0 kj/mol. Special properties of water are its high heat capacity and heat of vaporization, its ability to dissolve polar molecules, its cohesive and adhesive properties, and its dissociation into ions. Thermal properties of water at different temperatures. Evaporation Heat Capacity Water.
From www.ck12.org
Heating and Cooling Curves CK12 Foundation Evaporation Heat Capacity Water Thermal properties of water at different temperatures like density, freezing temperature, boiling temperature, latent heat of melting,. At room temperature the specific heat of liquid water is 4.18 j/ (g ⋅ ⋅ k) while the ethalpy of evaporation is 44.0 kj/mol. Special properties of water are its high heat capacity and heat of vaporization, its ability to dissolve polar molecules,. Evaporation Heat Capacity Water.
From www.researchgate.net
Evaporation capacity, pump work, and system efficiency with respect to Evaporation Heat Capacity Water 53 rows figures and tables showing how the properties of water changes along the boiling/condensation curve (vapor pressure,. Thermal properties of water at different temperatures like density, freezing temperature, boiling temperature, latent heat of melting,. At room temperature the specific heat of liquid water is 4.18 j/ (g ⋅ ⋅ k) while the ethalpy of evaporation is 44.0 kj/mol. Special. Evaporation Heat Capacity Water.
From gcsephysicsninja.com
42. How evaporation causes cooling Evaporation Heat Capacity Water At room temperature the specific heat of liquid water is 4.18 j/ (g ⋅ ⋅ k) while the ethalpy of evaporation is 44.0 kj/mol. Thermal properties of water at different temperatures like density, freezing temperature, boiling temperature, latent heat of melting,. I was wondering how to calculate the mass of water that evaporates if i were to expose the water. Evaporation Heat Capacity Water.
From shinestarpk.blogspot.com
Education World What Is The Evaporation? Evaporation Heat Capacity Water Special properties of water are its high heat capacity and heat of vaporization, its ability to dissolve polar molecules, its cohesive and adhesive properties, and its dissociation into ions. 53 rows figures and tables showing how the properties of water changes along the boiling/condensation curve (vapor pressure,. I was wondering how to calculate the mass of water that evaporates if. Evaporation Heat Capacity Water.
From sciencenotes.org
Evaporative Cooler How It Works and Examples Evaporation Heat Capacity Water Thermal properties of water at different temperatures like density, freezing temperature, boiling temperature, latent heat of melting,. Special properties of water are its high heat capacity and heat of vaporization, its ability to dissolve polar molecules, its cohesive and adhesive properties, and its dissociation into ions. 53 rows figures and tables showing how the properties of water changes along the. Evaporation Heat Capacity Water.
From www.saltworkconsultants.com
Physics of evaporation Evaporation Heat Capacity Water I was wondering how to calculate the mass of water that evaporates if i were to expose the water to some amount of heat given by q. At room temperature the specific heat of liquid water is 4.18 j/ (g ⋅ ⋅ k) while the ethalpy of evaporation is 44.0 kj/mol. Thermal properties of water at different temperatures like density,. Evaporation Heat Capacity Water.
From www.bbc.co.uk
Evaporation BBC Bitesize Evaporation Heat Capacity Water Thermal properties of water at different temperatures like density, freezing temperature, boiling temperature, latent heat of melting,. Special properties of water are its high heat capacity and heat of vaporization, its ability to dissolve polar molecules, its cohesive and adhesive properties, and its dissociation into ions. 53 rows figures and tables showing how the properties of water changes along the. Evaporation Heat Capacity Water.
From www.youtube.com
Evaporation Heat transfer coeff (DRAFT video) YouTube Evaporation Heat Capacity Water I was wondering how to calculate the mass of water that evaporates if i were to expose the water to some amount of heat given by q. 53 rows figures and tables showing how the properties of water changes along the boiling/condensation curve (vapor pressure,. At room temperature the specific heat of liquid water is 4.18 j/ (g ⋅ ⋅. Evaporation Heat Capacity Water.
From learningschooltrkesp5v.z22.web.core.windows.net
Water Evaporation Rate By Temperature Evaporation Heat Capacity Water At room temperature the specific heat of liquid water is 4.18 j/ (g ⋅ ⋅ k) while the ethalpy of evaporation is 44.0 kj/mol. Thermal properties of water at different temperatures like density, freezing temperature, boiling temperature, latent heat of melting,. Special properties of water are its high heat capacity and heat of vaporization, its ability to dissolve polar molecules,. Evaporation Heat Capacity Water.
From www.chemicals.co.uk
What is the Definition of Evaporation in Chemistry? Evaporation Heat Capacity Water I was wondering how to calculate the mass of water that evaporates if i were to expose the water to some amount of heat given by q. At room temperature the specific heat of liquid water is 4.18 j/ (g ⋅ ⋅ k) while the ethalpy of evaporation is 44.0 kj/mol. 53 rows figures and tables showing how the properties. Evaporation Heat Capacity Water.
From www.dreamstime.com
Boiling and Evaporation, Freezing and Melting Points of Water Stock Evaporation Heat Capacity Water 53 rows figures and tables showing how the properties of water changes along the boiling/condensation curve (vapor pressure,. Thermal properties of water at different temperatures like density, freezing temperature, boiling temperature, latent heat of melting,. I was wondering how to calculate the mass of water that evaporates if i were to expose the water to some amount of heat given. Evaporation Heat Capacity Water.
From www.slideserve.com
PPT Heat Capacity and Specific Heat Capacity PowerPoint Presentation Evaporation Heat Capacity Water Special properties of water are its high heat capacity and heat of vaporization, its ability to dissolve polar molecules, its cohesive and adhesive properties, and its dissociation into ions. Thermal properties of water at different temperatures like density, freezing temperature, boiling temperature, latent heat of melting,. At room temperature the specific heat of liquid water is 4.18 j/ (g ⋅. Evaporation Heat Capacity Water.
From www.researchgate.net
Energy balance diagram of a solar water evaporation system based on Evaporation Heat Capacity Water Thermal properties of water at different temperatures like density, freezing temperature, boiling temperature, latent heat of melting,. I was wondering how to calculate the mass of water that evaporates if i were to expose the water to some amount of heat given by q. At room temperature the specific heat of liquid water is 4.18 j/ (g ⋅ ⋅ k). Evaporation Heat Capacity Water.
From www.researchgate.net
a Schematic illustration of water evaporation process and mechanism. b Evaporation Heat Capacity Water I was wondering how to calculate the mass of water that evaporates if i were to expose the water to some amount of heat given by q. Thermal properties of water at different temperatures like density, freezing temperature, boiling temperature, latent heat of melting,. Special properties of water are its high heat capacity and heat of vaporization, its ability to. Evaporation Heat Capacity Water.
From www.bigditch.com.au
Evaporation management 101 Big Ditch Dam Company Evaporation Heat Capacity Water At room temperature the specific heat of liquid water is 4.18 j/ (g ⋅ ⋅ k) while the ethalpy of evaporation is 44.0 kj/mol. Thermal properties of water at different temperatures like density, freezing temperature, boiling temperature, latent heat of melting,. 53 rows figures and tables showing how the properties of water changes along the boiling/condensation curve (vapor pressure,. I. Evaporation Heat Capacity Water.
From www.expii.com
Heat Capacity of Water — Overview & Importance Expii Evaporation Heat Capacity Water I was wondering how to calculate the mass of water that evaporates if i were to expose the water to some amount of heat given by q. 53 rows figures and tables showing how the properties of water changes along the boiling/condensation curve (vapor pressure,. At room temperature the specific heat of liquid water is 4.18 j/ (g ⋅ ⋅. Evaporation Heat Capacity Water.
From www.scienceabc.com
Why Does Water Evaporate At Room Temperature? Evaporation Heat Capacity Water 53 rows figures and tables showing how the properties of water changes along the boiling/condensation curve (vapor pressure,. Thermal properties of water at different temperatures like density, freezing temperature, boiling temperature, latent heat of melting,. Special properties of water are its high heat capacity and heat of vaporization, its ability to dissolve polar molecules, its cohesive and adhesive properties, and. Evaporation Heat Capacity Water.
From www.slideserve.com
PPT Chapter 4 Water in the Atmosphere PowerPoint Presentation, free Evaporation Heat Capacity Water 53 rows figures and tables showing how the properties of water changes along the boiling/condensation curve (vapor pressure,. I was wondering how to calculate the mass of water that evaporates if i were to expose the water to some amount of heat given by q. Thermal properties of water at different temperatures like density, freezing temperature, boiling temperature, latent heat. Evaporation Heat Capacity Water.
From www.youtube.com
Evaporation and Condensation of Water Drop Simultaneous Heat and Mass Evaporation Heat Capacity Water At room temperature the specific heat of liquid water is 4.18 j/ (g ⋅ ⋅ k) while the ethalpy of evaporation is 44.0 kj/mol. 53 rows figures and tables showing how the properties of water changes along the boiling/condensation curve (vapor pressure,. Thermal properties of water at different temperatures like density, freezing temperature, boiling temperature, latent heat of melting,. Special. Evaporation Heat Capacity Water.
From thebiologyprimer.com
Atoms & Molecules echapter — The Biology Primer Evaporation Heat Capacity Water I was wondering how to calculate the mass of water that evaporates if i were to expose the water to some amount of heat given by q. 53 rows figures and tables showing how the properties of water changes along the boiling/condensation curve (vapor pressure,. Thermal properties of water at different temperatures like density, freezing temperature, boiling temperature, latent heat. Evaporation Heat Capacity Water.
From www.teachoo.com
How does Evaporation cause cooling? Explain (with Examples) Teachoo Evaporation Heat Capacity Water I was wondering how to calculate the mass of water that evaporates if i were to expose the water to some amount of heat given by q. 53 rows figures and tables showing how the properties of water changes along the boiling/condensation curve (vapor pressure,. Thermal properties of water at different temperatures like density, freezing temperature, boiling temperature, latent heat. Evaporation Heat Capacity Water.
From chem.libretexts.org
11.7 Heating Curve for Water Chemistry LibreTexts Evaporation Heat Capacity Water At room temperature the specific heat of liquid water is 4.18 j/ (g ⋅ ⋅ k) while the ethalpy of evaporation is 44.0 kj/mol. I was wondering how to calculate the mass of water that evaporates if i were to expose the water to some amount of heat given by q. Special properties of water are its high heat capacity. Evaporation Heat Capacity Water.
From geo.libretexts.org
3.4 Latent Heat and Heat Capacity Geosciences LibreTexts Evaporation Heat Capacity Water 53 rows figures and tables showing how the properties of water changes along the boiling/condensation curve (vapor pressure,. Special properties of water are its high heat capacity and heat of vaporization, its ability to dissolve polar molecules, its cohesive and adhesive properties, and its dissociation into ions. Thermal properties of water at different temperatures like density, freezing temperature, boiling temperature,. Evaporation Heat Capacity Water.
From present5.com
Thermodynamics I Te mperature Thermal Equilibrium and Evaporation Heat Capacity Water At room temperature the specific heat of liquid water is 4.18 j/ (g ⋅ ⋅ k) while the ethalpy of evaporation is 44.0 kj/mol. I was wondering how to calculate the mass of water that evaporates if i were to expose the water to some amount of heat given by q. Thermal properties of water at different temperatures like density,. Evaporation Heat Capacity Water.
From apollo.nvu.vsc.edu
Latent Heat of evaporation, fusion, and freezing Evaporation Heat Capacity Water At room temperature the specific heat of liquid water is 4.18 j/ (g ⋅ ⋅ k) while the ethalpy of evaporation is 44.0 kj/mol. I was wondering how to calculate the mass of water that evaporates if i were to expose the water to some amount of heat given by q. Thermal properties of water at different temperatures like density,. Evaporation Heat Capacity Water.
From www.researchgate.net
Temperature dependence of the latent heat of evaporation of water [43 Evaporation Heat Capacity Water I was wondering how to calculate the mass of water that evaporates if i were to expose the water to some amount of heat given by q. Thermal properties of water at different temperatures like density, freezing temperature, boiling temperature, latent heat of melting,. Special properties of water are its high heat capacity and heat of vaporization, its ability to. Evaporation Heat Capacity Water.
From www.slideserve.com
PPT GEU 0047 Meteorology Lecture 02 Heat Energy PowerPoint Evaporation Heat Capacity Water Thermal properties of water at different temperatures like density, freezing temperature, boiling temperature, latent heat of melting,. At room temperature the specific heat of liquid water is 4.18 j/ (g ⋅ ⋅ k) while the ethalpy of evaporation is 44.0 kj/mol. 53 rows figures and tables showing how the properties of water changes along the boiling/condensation curve (vapor pressure,. Special. Evaporation Heat Capacity Water.
From www.youtube.com
Lecture 4 Introduction to evaporation and latent heat YouTube Evaporation Heat Capacity Water Special properties of water are its high heat capacity and heat of vaporization, its ability to dissolve polar molecules, its cohesive and adhesive properties, and its dissociation into ions. Thermal properties of water at different temperatures like density, freezing temperature, boiling temperature, latent heat of melting,. I was wondering how to calculate the mass of water that evaporates if i. Evaporation Heat Capacity Water.
From www.toppr.com
The molar heat capacity of water at constant pressure is 75 JK 1 mol Evaporation Heat Capacity Water At room temperature the specific heat of liquid water is 4.18 j/ (g ⋅ ⋅ k) while the ethalpy of evaporation is 44.0 kj/mol. Special properties of water are its high heat capacity and heat of vaporization, its ability to dissolve polar molecules, its cohesive and adhesive properties, and its dissociation into ions. 53 rows figures and tables showing how. Evaporation Heat Capacity Water.
From www.researchgate.net
Schematic illustration of solardriven interfacial water evaporation Evaporation Heat Capacity Water I was wondering how to calculate the mass of water that evaporates if i were to expose the water to some amount of heat given by q. At room temperature the specific heat of liquid water is 4.18 j/ (g ⋅ ⋅ k) while the ethalpy of evaporation is 44.0 kj/mol. 53 rows figures and tables showing how the properties. Evaporation Heat Capacity Water.
From www.researchgate.net
Isobaric evaporation enthalpy of water, indicated by "0 g kg −1 ", and Evaporation Heat Capacity Water Special properties of water are its high heat capacity and heat of vaporization, its ability to dissolve polar molecules, its cohesive and adhesive properties, and its dissociation into ions. I was wondering how to calculate the mass of water that evaporates if i were to expose the water to some amount of heat given by q. At room temperature the. Evaporation Heat Capacity Water.
From www.youtube.com
Measuring the specific latent heat of vaporization of water YouTube Evaporation Heat Capacity Water I was wondering how to calculate the mass of water that evaporates if i were to expose the water to some amount of heat given by q. Special properties of water are its high heat capacity and heat of vaporization, its ability to dissolve polar molecules, its cohesive and adhesive properties, and its dissociation into ions. At room temperature the. Evaporation Heat Capacity Water.