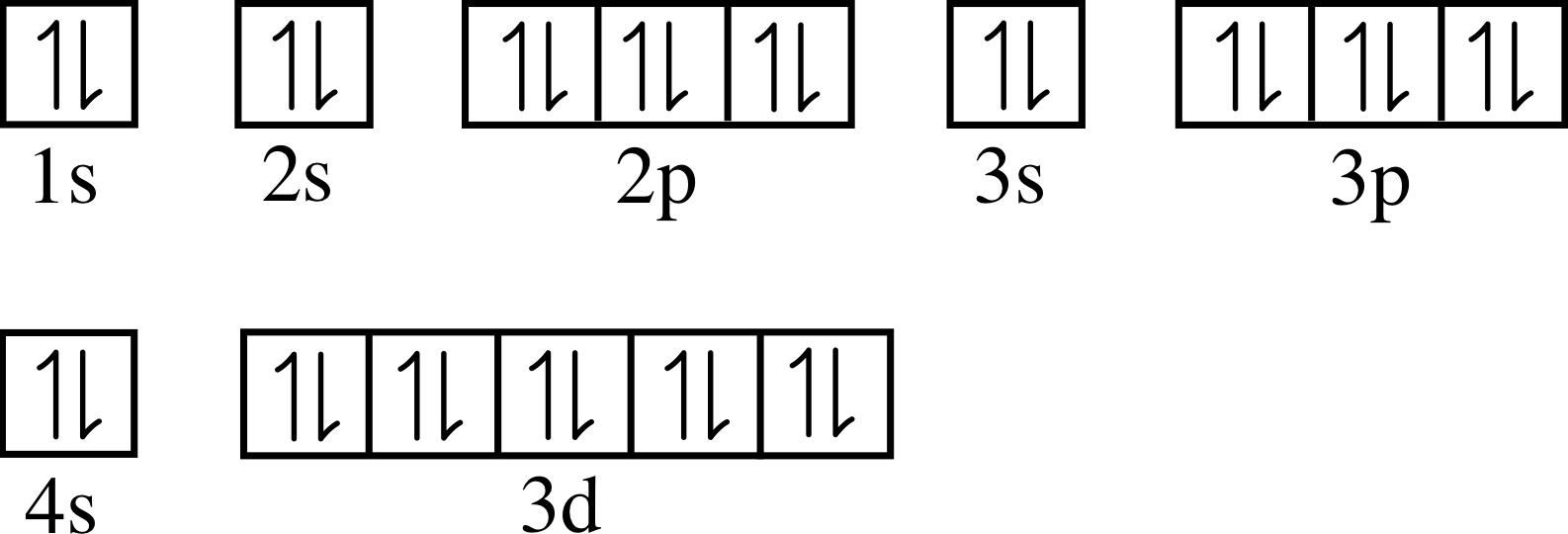
Zinc Electron Configuration 2 8 8 . electron configuration of zinc. The mg 2+ and zn 2+ ions are of the same size. Its atomic number is 30, so its complete electron configuration is 1s2 2s22p6 3s23p63d10 4s2. 119 rows electron configuration chart of all elements is mentioned in the table below. zinc is a chemical element of the periodic table with chemical symbol zn and atomic number 30 with an atomic weight of. find the atomic number of nitrogen (7) and use this electron configuration calculator to get a complete electron configuration. It has five stable isotopes. zinc electron configuration. The common zinc ore is sphalerite or zinc blende, which is a zinc sulfide mineral. Zinc is the 24th most abundant element present in the earth’s crust. This element has 4 energy levels and in its outermost shell it has 2 electrons. Its simplified electron configuration is [ar] 3d¹⁰ 4s². since the electronic configuration of zinc is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰, the valence electrons in zinc are.
from animalia-life.club
electron configuration of zinc. 119 rows electron configuration chart of all elements is mentioned in the table below. find the atomic number of nitrogen (7) and use this electron configuration calculator to get a complete electron configuration. since the electronic configuration of zinc is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰, the valence electrons in zinc are. Its atomic number is 30, so its complete electron configuration is 1s2 2s22p6 3s23p63d10 4s2. The common zinc ore is sphalerite or zinc blende, which is a zinc sulfide mineral. zinc electron configuration. This element has 4 energy levels and in its outermost shell it has 2 electrons. Zinc is the 24th most abundant element present in the earth’s crust. zinc is a chemical element of the periodic table with chemical symbol zn and atomic number 30 with an atomic weight of.
Zinc Electron Configuration
Zinc Electron Configuration 2 8 8 electron configuration of zinc. zinc is a chemical element of the periodic table with chemical symbol zn and atomic number 30 with an atomic weight of. zinc electron configuration. This element has 4 energy levels and in its outermost shell it has 2 electrons. Zinc is the 24th most abundant element present in the earth’s crust. The mg 2+ and zn 2+ ions are of the same size. since the electronic configuration of zinc is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰, the valence electrons in zinc are. Its simplified electron configuration is [ar] 3d¹⁰ 4s². 119 rows electron configuration chart of all elements is mentioned in the table below. electron configuration of zinc. It has five stable isotopes. find the atomic number of nitrogen (7) and use this electron configuration calculator to get a complete electron configuration. The common zinc ore is sphalerite or zinc blende, which is a zinc sulfide mineral. Its atomic number is 30, so its complete electron configuration is 1s2 2s22p6 3s23p63d10 4s2.
From animalia-life.club
Zinc Electron Configuration Zinc Electron Configuration 2 8 8 The common zinc ore is sphalerite or zinc blende, which is a zinc sulfide mineral. since the electronic configuration of zinc is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰, the valence electrons in zinc are. electron configuration of zinc. Its atomic number is 30, so its complete electron configuration is 1s2 2s22p6 3s23p63d10 4s2. Its simplified electron configuration. Zinc Electron Configuration 2 8 8.
From animalia-life.club
Zinc Electron Configuration Zinc Electron Configuration 2 8 8 119 rows electron configuration chart of all elements is mentioned in the table below. zinc electron configuration. The mg 2+ and zn 2+ ions are of the same size. It has five stable isotopes. Its atomic number is 30, so its complete electron configuration is 1s2 2s22p6 3s23p63d10 4s2. Its simplified electron configuration is [ar] 3d¹⁰ 4s². . Zinc Electron Configuration 2 8 8.
From animalia-life.club
Zinc Electron Configuration Zinc Electron Configuration 2 8 8 The common zinc ore is sphalerite or zinc blende, which is a zinc sulfide mineral. zinc electron configuration. Its simplified electron configuration is [ar] 3d¹⁰ 4s². electron configuration of zinc. find the atomic number of nitrogen (7) and use this electron configuration calculator to get a complete electron configuration. zinc is a chemical element of the. Zinc Electron Configuration 2 8 8.
From animalia-life.club
Zinc Electron Configuration Zinc Electron Configuration 2 8 8 The mg 2+ and zn 2+ ions are of the same size. Zinc is the 24th most abundant element present in the earth’s crust. zinc is a chemical element of the periodic table with chemical symbol zn and atomic number 30 with an atomic weight of. since the electronic configuration of zinc is 1s² 2s² 2p⁶ 3s² 3p⁶. Zinc Electron Configuration 2 8 8.
From www.alamy.es
Configuración de electrones del zinc. Ilustración de la estructura atómica y configuración de Zinc Electron Configuration 2 8 8 electron configuration of zinc. The mg 2+ and zn 2+ ions are of the same size. find the atomic number of nitrogen (7) and use this electron configuration calculator to get a complete electron configuration. zinc is a chemical element of the periodic table with chemical symbol zn and atomic number 30 with an atomic weight of.. Zinc Electron Configuration 2 8 8.
From www.freepik.com
Free Vector A zinc atom diagram Zinc Electron Configuration 2 8 8 119 rows electron configuration chart of all elements is mentioned in the table below. Zinc is the 24th most abundant element present in the earth’s crust. This element has 4 energy levels and in its outermost shell it has 2 electrons. zinc electron configuration. electron configuration of zinc. The common zinc ore is sphalerite or zinc blende,. Zinc Electron Configuration 2 8 8.
From dxonyecim.blob.core.windows.net
Zn Electron Configuration Diagram at Beatrice Fitch blog Zinc Electron Configuration 2 8 8 electron configuration of zinc. Its simplified electron configuration is [ar] 3d¹⁰ 4s². The common zinc ore is sphalerite or zinc blende, which is a zinc sulfide mineral. The mg 2+ and zn 2+ ions are of the same size. zinc electron configuration. Zinc is the 24th most abundant element present in the earth’s crust. Its atomic number is. Zinc Electron Configuration 2 8 8.
From artoflifestyles.blogspot.com
Electron Configuration For Zn And Zn2+ (Zinc And Zinc Ion) YouTube Master News Zinc Electron Configuration 2 8 8 find the atomic number of nitrogen (7) and use this electron configuration calculator to get a complete electron configuration. The mg 2+ and zn 2+ ions are of the same size. The common zinc ore is sphalerite or zinc blende, which is a zinc sulfide mineral. Its simplified electron configuration is [ar] 3d¹⁰ 4s². zinc electron configuration. . Zinc Electron Configuration 2 8 8.
From golace.blogspot.com
zinc orbital diagram Golace Zinc Electron Configuration 2 8 8 since the electronic configuration of zinc is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰, the valence electrons in zinc are. 119 rows electron configuration chart of all elements is mentioned in the table below. find the atomic number of nitrogen (7) and use this electron configuration calculator to get a complete electron configuration. The mg 2+ and. Zinc Electron Configuration 2 8 8.
From wiringlistvoltairean.z14.web.core.windows.net
Full Electron Configuration For Zinc Zinc Electron Configuration 2 8 8 zinc electron configuration. The mg 2+ and zn 2+ ions are of the same size. The common zinc ore is sphalerite or zinc blende, which is a zinc sulfide mineral. since the electronic configuration of zinc is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰, the valence electrons in zinc are. Its simplified electron configuration is [ar] 3d¹⁰ 4s².. Zinc Electron Configuration 2 8 8.
From www.alamy.com
Zinc (Zn). Diagram of the nuclear composition and electron configuration of an atom of zinc64 Zinc Electron Configuration 2 8 8 zinc electron configuration. Its atomic number is 30, so its complete electron configuration is 1s2 2s22p6 3s23p63d10 4s2. This element has 4 energy levels and in its outermost shell it has 2 electrons. zinc is a chemical element of the periodic table with chemical symbol zn and atomic number 30 with an atomic weight of. find the. Zinc Electron Configuration 2 8 8.
From animalia-life.club
Zinc Electron Configuration Zinc Electron Configuration 2 8 8 find the atomic number of nitrogen (7) and use this electron configuration calculator to get a complete electron configuration. zinc electron configuration. This element has 4 energy levels and in its outermost shell it has 2 electrons. The common zinc ore is sphalerite or zinc blende, which is a zinc sulfide mineral. Its simplified electron configuration is [ar]. Zinc Electron Configuration 2 8 8.
From www.coursehero.com
[Solved] . WHAT TO DO 1. Illustrate the electron configuration of zinc... Course Hero Zinc Electron Configuration 2 8 8 The mg 2+ and zn 2+ ions are of the same size. find the atomic number of nitrogen (7) and use this electron configuration calculator to get a complete electron configuration. electron configuration of zinc. since the electronic configuration of zinc is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰, the valence electrons in zinc are. It has. Zinc Electron Configuration 2 8 8.
From www.britannica.com
zinc Properties, Uses, & Facts Britannica Zinc Electron Configuration 2 8 8 since the electronic configuration of zinc is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰, the valence electrons in zinc are. zinc electron configuration. electron configuration of zinc. It has five stable isotopes. zinc is a chemical element of the periodic table with chemical symbol zn and atomic number 30 with an atomic weight of. The common. Zinc Electron Configuration 2 8 8.
From exosblltn.blob.core.windows.net
Zinc Oxide Electron Configuration at Cherry Lee blog Zinc Electron Configuration 2 8 8 119 rows electron configuration chart of all elements is mentioned in the table below. since the electronic configuration of zinc is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰, the valence electrons in zinc are. zinc electron configuration. Its atomic number is 30, so its complete electron configuration is 1s2 2s22p6 3s23p63d10 4s2. electron configuration of zinc.. Zinc Electron Configuration 2 8 8.
From animalia-life.club
Zinc Electron Configuration Zinc Electron Configuration 2 8 8 Zinc is the 24th most abundant element present in the earth’s crust. 119 rows electron configuration chart of all elements is mentioned in the table below. The common zinc ore is sphalerite or zinc blende, which is a zinc sulfide mineral. The mg 2+ and zn 2+ ions are of the same size. zinc is a chemical element. Zinc Electron Configuration 2 8 8.
From dxonyecim.blob.core.windows.net
Zn Electron Configuration Diagram at Beatrice Fitch blog Zinc Electron Configuration 2 8 8 Its atomic number is 30, so its complete electron configuration is 1s2 2s22p6 3s23p63d10 4s2. electron configuration of zinc. since the electronic configuration of zinc is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰, the valence electrons in zinc are. zinc electron configuration. zinc is a chemical element of the periodic table with chemical symbol zn and. Zinc Electron Configuration 2 8 8.
From www.youtube.com
How to Find the Valence Electrons for Zinc (Zn) YouTube Zinc Electron Configuration 2 8 8 This element has 4 energy levels and in its outermost shell it has 2 electrons. It has five stable isotopes. zinc electron configuration. Zinc is the 24th most abundant element present in the earth’s crust. zinc is a chemical element of the periodic table with chemical symbol zn and atomic number 30 with an atomic weight of. Its. Zinc Electron Configuration 2 8 8.
From www.thoughtco.com
Atom Diagrams Electron Configurations of the Elements Zinc Electron Configuration 2 8 8 Its atomic number is 30, so its complete electron configuration is 1s2 2s22p6 3s23p63d10 4s2. since the electronic configuration of zinc is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰, the valence electrons in zinc are. zinc is a chemical element of the periodic table with chemical symbol zn and atomic number 30 with an atomic weight of. Zinc. Zinc Electron Configuration 2 8 8.
From animalia-life.club
Zinc Electron Configuration Zinc Electron Configuration 2 8 8 Its atomic number is 30, so its complete electron configuration is 1s2 2s22p6 3s23p63d10 4s2. since the electronic configuration of zinc is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰, the valence electrons in zinc are. The common zinc ore is sphalerite or zinc blende, which is a zinc sulfide mineral. This element has 4 energy levels and in its. Zinc Electron Configuration 2 8 8.
From www.youtube.com
Electron Configuration of Zinc Zn Lesson YouTube Zinc Electron Configuration 2 8 8 It has five stable isotopes. zinc electron configuration. 119 rows electron configuration chart of all elements is mentioned in the table below. find the atomic number of nitrogen (7) and use this electron configuration calculator to get a complete electron configuration. This element has 4 energy levels and in its outermost shell it has 2 electrons. . Zinc Electron Configuration 2 8 8.
From animalia-life.club
Zinc Electron Configuration Zinc Electron Configuration 2 8 8 The mg 2+ and zn 2+ ions are of the same size. zinc is a chemical element of the periodic table with chemical symbol zn and atomic number 30 with an atomic weight of. This element has 4 energy levels and in its outermost shell it has 2 electrons. Its atomic number is 30, so its complete electron configuration. Zinc Electron Configuration 2 8 8.
From www.chegg.com
Solved 4 a. Write the electron configuration for the Zinc Zinc Electron Configuration 2 8 8 The mg 2+ and zn 2+ ions are of the same size. Its atomic number is 30, so its complete electron configuration is 1s2 2s22p6 3s23p63d10 4s2. 119 rows electron configuration chart of all elements is mentioned in the table below. The common zinc ore is sphalerite or zinc blende, which is a zinc sulfide mineral. This element has. Zinc Electron Configuration 2 8 8.
From www.alamy.com
Zn Zinc, Periodic Table of the Elements, Shell Structure of Zinc Electrons per energy level Zinc Electron Configuration 2 8 8 Zinc is the 24th most abundant element present in the earth’s crust. This element has 4 energy levels and in its outermost shell it has 2 electrons. zinc is a chemical element of the periodic table with chemical symbol zn and atomic number 30 with an atomic weight of. 119 rows electron configuration chart of all elements is. Zinc Electron Configuration 2 8 8.
From dxonyecim.blob.core.windows.net
Zn Electron Configuration Diagram at Beatrice Fitch blog Zinc Electron Configuration 2 8 8 The common zinc ore is sphalerite or zinc blende, which is a zinc sulfide mineral. zinc electron configuration. 119 rows electron configuration chart of all elements is mentioned in the table below. find the atomic number of nitrogen (7) and use this electron configuration calculator to get a complete electron configuration. The mg 2+ and zn 2+. Zinc Electron Configuration 2 8 8.
From www.istockphoto.com
Electron Configuration For Zinc Stock Photos, Pictures & RoyaltyFree Images iStock Zinc Electron Configuration 2 8 8 The mg 2+ and zn 2+ ions are of the same size. Its simplified electron configuration is [ar] 3d¹⁰ 4s². This element has 4 energy levels and in its outermost shell it has 2 electrons. zinc electron configuration. find the atomic number of nitrogen (7) and use this electron configuration calculator to get a complete electron configuration. It. Zinc Electron Configuration 2 8 8.
From ar.inspiredpencil.com
Zinc Electron Configuration Zinc Electron Configuration 2 8 8 Its simplified electron configuration is [ar] 3d¹⁰ 4s². electron configuration of zinc. Zinc is the 24th most abundant element present in the earth’s crust. since the electronic configuration of zinc is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰, the valence electrons in zinc are. zinc is a chemical element of the periodic table with chemical symbol zn. Zinc Electron Configuration 2 8 8.
From www.youtube.com
How to find Protons & Electrons for the Zn 2+ (Zinc ion) YouTube Zinc Electron Configuration 2 8 8 The common zinc ore is sphalerite or zinc blende, which is a zinc sulfide mineral. electron configuration of zinc. zinc is a chemical element of the periodic table with chemical symbol zn and atomic number 30 with an atomic weight of. It has five stable isotopes. The mg 2+ and zn 2+ ions are of the same size.. Zinc Electron Configuration 2 8 8.
From www.sciencefacts.net
Electron Configuration Definition, Examples, Chart, and Diagram Zinc Electron Configuration 2 8 8 Its simplified electron configuration is [ar] 3d¹⁰ 4s². It has five stable isotopes. Its atomic number is 30, so its complete electron configuration is 1s2 2s22p6 3s23p63d10 4s2. Zinc is the 24th most abundant element present in the earth’s crust. This element has 4 energy levels and in its outermost shell it has 2 electrons. The mg 2+ and zn. Zinc Electron Configuration 2 8 8.
From educationhirple.z21.web.core.windows.net
What Is Zinc Electron Configuration Zinc Electron Configuration 2 8 8 electron configuration of zinc. The mg 2+ and zn 2+ ions are of the same size. find the atomic number of nitrogen (7) and use this electron configuration calculator to get a complete electron configuration. Zinc is the 24th most abundant element present in the earth’s crust. Its atomic number is 30, so its complete electron configuration is. Zinc Electron Configuration 2 8 8.
From stock.adobe.com
Bohr model representation of the zinc atom, number 30 and symbol Zn. Conceptual vector Zinc Electron Configuration 2 8 8 The mg 2+ and zn 2+ ions are of the same size. zinc electron configuration. It has five stable isotopes. 119 rows electron configuration chart of all elements is mentioned in the table below. since the electronic configuration of zinc is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰, the valence electrons in zinc are. The common zinc. Zinc Electron Configuration 2 8 8.
From www.youtube.com
zinc electronic configuration How to Write Zinc electronic configuration YouTube Zinc Electron Configuration 2 8 8 since the electronic configuration of zinc is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰, the valence electrons in zinc are. zinc is a chemical element of the periodic table with chemical symbol zn and atomic number 30 with an atomic weight of. Zinc is the 24th most abundant element present in the earth’s crust. This element has 4. Zinc Electron Configuration 2 8 8.
From www.youtube.com
Electron configuration, the 2, 8, 8, 2 rule. What are groups and what are periods. YouTube Zinc Electron Configuration 2 8 8 Its atomic number is 30, so its complete electron configuration is 1s2 2s22p6 3s23p63d10 4s2. zinc is a chemical element of the periodic table with chemical symbol zn and atomic number 30 with an atomic weight of. The mg 2+ and zn 2+ ions are of the same size. This element has 4 energy levels and in its outermost. Zinc Electron Configuration 2 8 8.
From periodictableguide.com
Zinc (Zn) Periodic Table (Element Information & More) Zinc Electron Configuration 2 8 8 This element has 4 energy levels and in its outermost shell it has 2 electrons. 119 rows electron configuration chart of all elements is mentioned in the table below. electron configuration of zinc. The common zinc ore is sphalerite or zinc blende, which is a zinc sulfide mineral. It has five stable isotopes. Zinc is the 24th most. Zinc Electron Configuration 2 8 8.
From material-properties.org
Zinc Periodic Table and Atomic Properties Zinc Electron Configuration 2 8 8 electron configuration of zinc. zinc is a chemical element of the periodic table with chemical symbol zn and atomic number 30 with an atomic weight of. Its atomic number is 30, so its complete electron configuration is 1s2 2s22p6 3s23p63d10 4s2. The common zinc ore is sphalerite or zinc blende, which is a zinc sulfide mineral. since. Zinc Electron Configuration 2 8 8.