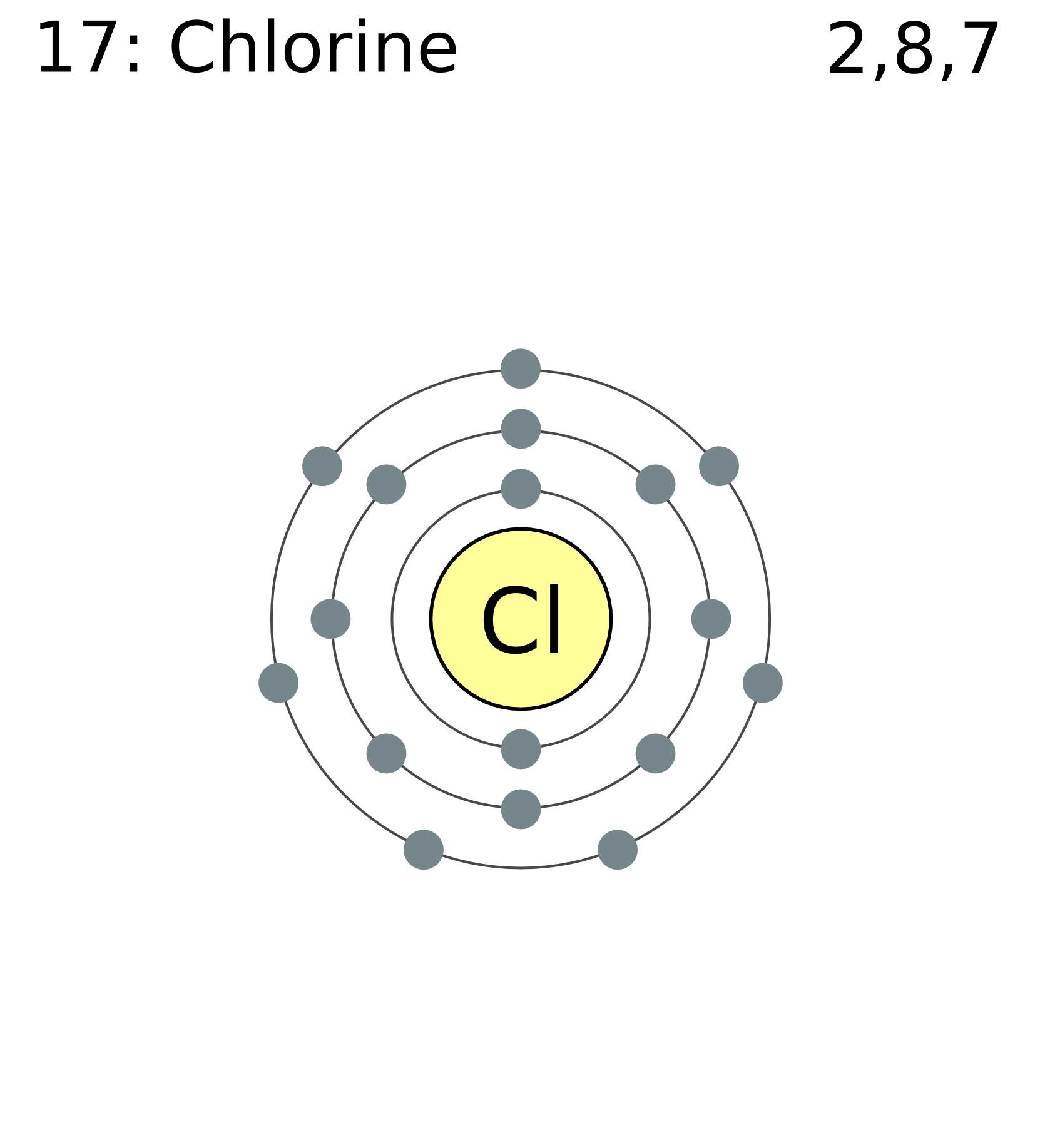
Chlorine Valence Shell Electron Configuration . A full valence shell is the most stable electron. Chlorine has an electron configuration of. the chlorine atom has the same electron configuration in the valence shell, but because the entering electron is. It is an extremely reactive element and a strong oxidising. the valence electron configuration for chlorine is s2p5. electron configuration of chlorine is [ne] 3s2 3p5. find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. in order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there.
from commons.wikimedia.org
A full valence shell is the most stable electron. Chlorine has an electron configuration of. the valence electron configuration for chlorine is s2p5. in order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there. It is an extremely reactive element and a strong oxidising. electron configuration of chlorine is [ne] 3s2 3p5. find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. the chlorine atom has the same electron configuration in the valence shell, but because the entering electron is.
FileElectron shell 017 chlorine.png Wikimedia Commons
Chlorine Valence Shell Electron Configuration group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. in order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there. electron configuration of chlorine is [ne] 3s2 3p5. A full valence shell is the most stable electron. find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. the valence electron configuration for chlorine is s2p5. Chlorine has an electron configuration of. the chlorine atom has the same electron configuration in the valence shell, but because the entering electron is. group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. It is an extremely reactive element and a strong oxidising.
From studiousguy.com
Chlorine (Cl) Properties & Uses StudiousGuy Chlorine Valence Shell Electron Configuration the valence electron configuration for chlorine is s2p5. group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. Chlorine has an electron configuration of. find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. the chlorine atom has the same. Chlorine Valence Shell Electron Configuration.
From womackthille.blogspot.com
Expanded Electron Configuration of Chlorine Womack Thille Chlorine Valence Shell Electron Configuration It is an extremely reactive element and a strong oxidising. electron configuration of chlorine is [ne] 3s2 3p5. Chlorine has an electron configuration of. group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. find the full electronic configuration and valence electrons of any periodic element using this. Chlorine Valence Shell Electron Configuration.
From www.thoughtco.com
Atoms Diagrams Electron Configurations of Elements Chlorine Valence Shell Electron Configuration electron configuration of chlorine is [ne] 3s2 3p5. Chlorine has an electron configuration of. in order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there. It is an extremely reactive element and a strong oxidising. group 18 elements (helium, neon, and argon are shown in figure. Chlorine Valence Shell Electron Configuration.
From www.britannica.com
Halogen Elements, Examples, Properties, Uses, & Facts Britannica Chlorine Valence Shell Electron Configuration It is an extremely reactive element and a strong oxidising. the valence electron configuration for chlorine is s2p5. in order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there. electron configuration of chlorine is [ne] 3s2 3p5. A full valence shell is the most stable electron.. Chlorine Valence Shell Electron Configuration.
From valenceelectrons.com
Electron Configuration for Chlorine (Cl) Full Explanation Chlorine Valence Shell Electron Configuration It is an extremely reactive element and a strong oxidising. A full valence shell is the most stable electron. the chlorine atom has the same electron configuration in the valence shell, but because the entering electron is. the valence electron configuration for chlorine is s2p5. in order to write the chlorine electron configuration we first need to. Chlorine Valence Shell Electron Configuration.
From www.toppr.com
What is the valence shell electronic configuration of chlorine atom in Chlorine Valence Shell Electron Configuration Chlorine has an electron configuration of. the chlorine atom has the same electron configuration in the valence shell, but because the entering electron is. the valence electron configuration for chlorine is s2p5. It is an extremely reactive element and a strong oxidising. electron configuration of chlorine is [ne] 3s2 3p5. find the full electronic configuration and. Chlorine Valence Shell Electron Configuration.
From www.newtondesk.com
Chlorine Cl (Element 17) of Periodic Table Newton Desk Chlorine Valence Shell Electron Configuration It is an extremely reactive element and a strong oxidising. find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. electron configuration of chlorine is [ne] 3s2 3p5. in order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there.. Chlorine Valence Shell Electron Configuration.
From www.ck12.org
Valence Electrons CK12 Foundation Chlorine Valence Shell Electron Configuration It is an extremely reactive element and a strong oxidising. group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. A full valence shell is the most stable electron. Chlorine has an electron configuration of. electron configuration of chlorine is [ne] 3s2 3p5. find the full electronic configuration. Chlorine Valence Shell Electron Configuration.
From 88guru.com
Electron Configuration Rules, Example and Diagram 88Guru Chlorine Valence Shell Electron Configuration It is an extremely reactive element and a strong oxidising. group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. the valence electron configuration for chlorine is s2p5. electron configuration of chlorine is [ne] 3s2 3p5. the chlorine atom has the same electron configuration in the valence. Chlorine Valence Shell Electron Configuration.
From byjus.com
In the electron dot structure, the valence shell electrons are Chlorine Valence Shell Electron Configuration in order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there. It is an extremely reactive element and a strong oxidising. the chlorine atom has the same electron configuration in the valence shell, but because the entering electron is. Chlorine has an electron configuration of. group. Chlorine Valence Shell Electron Configuration.
From chemistry291.blogspot.com
What Is the Chlorine(Cl) Electron Configuration? Chlorine Valence Shell Electron Configuration electron configuration of chlorine is [ne] 3s2 3p5. It is an extremely reactive element and a strong oxidising. group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. A full valence. Chlorine Valence Shell Electron Configuration.
From scheme360.net
Chlorine Electron Shell Diagram Chlorine Valence Shell Electron Configuration the chlorine atom has the same electron configuration in the valence shell, but because the entering electron is. find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. electron configuration. Chlorine Valence Shell Electron Configuration.
From exokabuml.blob.core.windows.net
Chlorine Electrons Neutrons And Protons at Wanda Palm blog Chlorine Valence Shell Electron Configuration the chlorine atom has the same electron configuration in the valence shell, but because the entering electron is. find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. in order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there.. Chlorine Valence Shell Electron Configuration.
From valenceelectrons.com
How Many Valence Electrons Does Chlorine (Cl) Have? Chlorine Valence Shell Electron Configuration the chlorine atom has the same electron configuration in the valence shell, but because the entering electron is. electron configuration of chlorine is [ne] 3s2 3p5. the valence electron configuration for chlorine is s2p5. in order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there.. Chlorine Valence Shell Electron Configuration.
From schematicdatavenin77.z5.web.core.windows.net
Dot Diagram For Chlorine Chlorine Valence Shell Electron Configuration find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. in order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there. the chlorine atom has the same electron configuration in the valence shell, but because the entering electron is.. Chlorine Valence Shell Electron Configuration.
From dxoawsdru.blob.core.windows.net
The Nonmetal Chlorine Electron Configuration at Adrian Tarry blog Chlorine Valence Shell Electron Configuration the valence electron configuration for chlorine is s2p5. electron configuration of chlorine is [ne] 3s2 3p5. find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. It is an extremely reactive element and a strong oxidising. in order to write the chlorine electron configuration we first need to know. Chlorine Valence Shell Electron Configuration.
From www.sciencefacts.net
Electron Configuration Definition, Examples, Chart, and Diagram Chlorine Valence Shell Electron Configuration electron configuration of chlorine is [ne] 3s2 3p5. the valence electron configuration for chlorine is s2p5. Chlorine has an electron configuration of. the chlorine atom has the same electron configuration in the valence shell, but because the entering electron is. find the full electronic configuration and valence electrons of any periodic element using this electron configuration. Chlorine Valence Shell Electron Configuration.
From sciencenotes.org
Chlorine Facts Chlorine Valence Shell Electron Configuration A full valence shell is the most stable electron. group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. the chlorine atom has the same electron configuration in the valence shell, but because the entering electron is. in order to write the chlorine electron configuration we first need. Chlorine Valence Shell Electron Configuration.
From mungfali.com
Valence Electron Shell Diagram Chlorine Valence Shell Electron Configuration Chlorine has an electron configuration of. group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. It is an extremely reactive element and a strong oxidising. the chlorine atom has the same electron configuration in the valence shell, but because the entering electron is. electron configuration of chlorine. Chlorine Valence Shell Electron Configuration.
From topblogtenz.com
Chlorine Orbital diagram, Electron configuration, and Valence electrons Chlorine Valence Shell Electron Configuration Chlorine has an electron configuration of. the chlorine atom has the same electron configuration in the valence shell, but because the entering electron is. the valence electron configuration for chlorine is s2p5. It is an extremely reactive element and a strong oxidising. group 18 elements (helium, neon, and argon are shown in figure 2) have a full. Chlorine Valence Shell Electron Configuration.
From www.slideserve.com
PPT Electron Energy Levels and Configurations PowerPoint Presentation Chlorine Valence Shell Electron Configuration find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. electron configuration of chlorine is [ne] 3s2 3p5. It is an extremely reactive element and a strong oxidising. Chlorine has an. Chlorine Valence Shell Electron Configuration.
From byjus.com
How will you find the valence of Chlorine, Sulphur and Magnesium? Chlorine Valence Shell Electron Configuration find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. It is an extremely reactive element and a strong oxidising. group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. in order to write the chlorine electron configuration we first need. Chlorine Valence Shell Electron Configuration.
From www.shutterstock.com
Atom Chlorine This Diagram Shows Electron Stock Vector 328668782 Chlorine Valence Shell Electron Configuration the chlorine atom has the same electron configuration in the valence shell, but because the entering electron is. group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. in order to write the chlorine electron configuration we first need to know the number of electrons for the cl. Chlorine Valence Shell Electron Configuration.
From chemistry291.blogspot.com
How Many Valence Electrons Does chlorine Have?number of valence Chlorine Valence Shell Electron Configuration the valence electron configuration for chlorine is s2p5. It is an extremely reactive element and a strong oxidising. find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. A full valence shell is the most stable electron. Chlorine has an electron configuration of. group 18 elements (helium, neon, and argon. Chlorine Valence Shell Electron Configuration.
From periodictable.me
Chlorine Electron Configuration (Cl) with Orbital Diagram Chlorine Valence Shell Electron Configuration group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. electron configuration of chlorine is [ne] 3s2 3p5. A full valence shell is the most stable electron. in order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom. Chlorine Valence Shell Electron Configuration.
From www.chegg.com
Solved What is the electron configuration for Cl, chlorine, Chlorine Valence Shell Electron Configuration the valence electron configuration for chlorine is s2p5. electron configuration of chlorine is [ne] 3s2 3p5. group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. in order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom. Chlorine Valence Shell Electron Configuration.
From commons.wikimedia.org
FileElectron shell 017 chlorine.png Wikimedia Commons Chlorine Valence Shell Electron Configuration group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. Chlorine has an electron configuration of. A full valence shell is the most stable electron. electron configuration of chlorine is [ne] 3s2 3p5. It is an extremely reactive element and a strong oxidising. the chlorine atom has the. Chlorine Valence Shell Electron Configuration.
From utedzz.blogspot.com
Periodic Table Chlorine Atomic Number Periodic Table Timeline Chlorine Valence Shell Electron Configuration Chlorine has an electron configuration of. find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. A full valence shell is the most stable electron. electron configuration of chlorine is [ne] 3s2 3p5. the valence electron configuration for chlorine is s2p5. group 18 elements (helium, neon, and argon are. Chlorine Valence Shell Electron Configuration.
From elchoroukhost.net
Chlorine Periodic Table Electron Configuration Elcho Table Chlorine Valence Shell Electron Configuration in order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there. It is an extremely reactive element and a strong oxidising. Chlorine has an electron configuration of. find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. A full valence. Chlorine Valence Shell Electron Configuration.
From periodictable.me
Chlorine Electron Configuration (Cl) with Orbital Diagram Chlorine Valence Shell Electron Configuration electron configuration of chlorine is [ne] 3s2 3p5. group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. Chlorine has an electron configuration of. It is an extremely reactive element and a strong oxidising. the chlorine atom has the same electron configuration in the valence shell, but because. Chlorine Valence Shell Electron Configuration.
From www.webelements.com
Elements Periodic Table » Chlorine » properties of free atoms Chlorine Valence Shell Electron Configuration electron configuration of chlorine is [ne] 3s2 3p5. find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. the chlorine atom has the same electron configuration in the valence shell, but because the entering electron is. the valence electron configuration for chlorine is s2p5. Chlorine has an electron configuration. Chlorine Valence Shell Electron Configuration.
From imgbin.com
Electron Configuration Aufbau Principle Valence Electron Chlorine PNG Chlorine Valence Shell Electron Configuration group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. the chlorine atom has the same electron configuration in the valence shell, but because the entering electron is. the valence electron configuration for chlorine is s2p5. A full valence shell is the most stable electron. electron configuration. Chlorine Valence Shell Electron Configuration.
From www.alamy.com
Chlorine (Cl). Diagram of the nuclear composition, electron Chlorine Valence Shell Electron Configuration It is an extremely reactive element and a strong oxidising. in order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there. the valence electron configuration for chlorine is s2p5. Chlorine has an electron configuration of. group 18 elements (helium, neon, and argon are shown in figure. Chlorine Valence Shell Electron Configuration.
From www.sciencephoto.com
Chlorine electron configuration Stock Image C029/5025 Science Chlorine Valence Shell Electron Configuration the valence electron configuration for chlorine is s2p5. It is an extremely reactive element and a strong oxidising. find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. in order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there.. Chlorine Valence Shell Electron Configuration.
From www.vectorstock.com
Diagram representation of the element chlorine Vector Image Chlorine Valence Shell Electron Configuration A full valence shell is the most stable electron. the valence electron configuration for chlorine is s2p5. It is an extremely reactive element and a strong oxidising. find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. in order to write the chlorine electron configuration we first need to know. Chlorine Valence Shell Electron Configuration.