From www.slideserve.com
PPT Elements and Compounds PowerPoint Presentation, free download Define Mixture Law Raoult's law states that the partial vapor pressure of a component of an ideal mixture is the vapor pressure of the pure component multiplied by its mole fraction. Raoult’s law states that a solvent’s partial vapour pressure in a solution (or mixture) is equal or identical to the vapour pressure of the pure solvent multiplied by its mole fraction. Raoult's. Define Mixture Law.
From www.slideserve.com
PPT Matter PowerPoint Presentation, free download ID2061285 Define Mixture Law This chapter discusses composition variables and partial molar quantities of mixtures in which no chemical reaction is occurring. Raoult's law states that the vapor pressure of a solvent above a solution is equal to the vapor pressure of the pure solvent at the same temperature scaled by the mole fraction of. The partial vapor pressure of a component in a. Define Mixture Law.
From en.wikipedia.org
Homogeneous and heterogeneous mixtures Wikipedia Define Mixture Law Raoult’s law states that a solvent’s partial vapour pressure in a solution (or mixture) is equal or identical to the vapour pressure of the pure solvent multiplied by its mole fraction. Raoult's law states that the partial vapor pressure of a component of an ideal mixture is the vapor pressure of the pure component multiplied by its mole fraction. Raoult's. Define Mixture Law.
From www.slideserve.com
PPT Mixtures PowerPoint Presentation, free download ID1978051 Define Mixture Law The partial vapor pressure of a component in a mixture is equal to the vapor pressure of the pure. Raoult's law states that the vapor pressure of a solvent above a solution is equal to the vapor pressure of the pure solvent at the same temperature scaled by the mole fraction of. Raoult’s law states that a solvent’s partial vapour. Define Mixture Law.
From www.sliderbase.com
Elements compounds and mixtures Presentation Chemistry Define Mixture Law This chapter discusses composition variables and partial molar quantities of mixtures in which no chemical reaction is occurring. Raoult's law states that the partial vapor pressure of a component of an ideal mixture is the vapor pressure of the pure component multiplied by its mole fraction. (rule of mixtures) properties of binary mixtures lie between the corresponding properties of the. Define Mixture Law.
From www.vedantu.com
Chemical Mixtures Learn Definition, Types and Examples Define Mixture Law The partial vapor pressure of a component in a mixture is equal to the vapor pressure of the pure. This chapter discusses composition variables and partial molar quantities of mixtures in which no chemical reaction is occurring. Raoult’s law states that a solvent’s partial vapour pressure in a solution (or mixture) is equal or identical to the vapour pressure of. Define Mixture Law.
From www.youtube.com
What is a mixture and its types? II Homogeneous mixture and Define Mixture Law (rule of mixtures) properties of binary mixtures lie between the corresponding properties of the pure components and are. Raoult's law states that the vapor pressure of a solvent above a solution is equal to the vapor pressure of the pure solvent at the same temperature scaled by the mole fraction of. This chapter discusses composition variables and partial molar quantities. Define Mixture Law.
From studylib.net
Elements, Compounds, Mixtures, Law of Define Mixture Law (rule of mixtures) properties of binary mixtures lie between the corresponding properties of the pure components and are. Raoult's law states that the partial vapor pressure of a component of an ideal mixture is the vapor pressure of the pure component multiplied by its mole fraction. Raoult’s law states that a solvent’s partial vapour pressure in a solution (or mixture). Define Mixture Law.
From www.slideshare.net
Real life chemistry Define Mixture Law Raoult's law states that the vapor pressure of a solvent above a solution is equal to the vapor pressure of the pure solvent at the same temperature scaled by the mole fraction of. The partial vapor pressure of a component in a mixture is equal to the vapor pressure of the pure. Raoult’s law states that a solvent’s partial vapour. Define Mixture Law.
From www.slideserve.com
PPT Classification of Matter PowerPoint Presentation, free download Define Mixture Law This chapter discusses composition variables and partial molar quantities of mixtures in which no chemical reaction is occurring. (rule of mixtures) properties of binary mixtures lie between the corresponding properties of the pure components and are. Raoult's law states that the partial vapor pressure of a component of an ideal mixture is the vapor pressure of the pure component multiplied. Define Mixture Law.
From unacademy.com
Science Class 9 Pure Substance vs Mixture UPSC Note on Science Class Define Mixture Law Raoult's law states that the vapor pressure of a solvent above a solution is equal to the vapor pressure of the pure solvent at the same temperature scaled by the mole fraction of. This chapter discusses composition variables and partial molar quantities of mixtures in which no chemical reaction is occurring. Raoult’s law states that a solvent’s partial vapour pressure. Define Mixture Law.
From www.geeksforgeeks.org
What is a Mixture? Definition, Properties, Examples, Types and FAQs Define Mixture Law This chapter discusses composition variables and partial molar quantities of mixtures in which no chemical reaction is occurring. (rule of mixtures) properties of binary mixtures lie between the corresponding properties of the pure components and are. Raoult’s law states that a solvent’s partial vapour pressure in a solution (or mixture) is equal or identical to the vapour pressure of the. Define Mixture Law.
From www.slideserve.com
PPT Separating Mixtures PowerPoint Presentation, free download ID Define Mixture Law (rule of mixtures) properties of binary mixtures lie between the corresponding properties of the pure components and are. Raoult's law states that the vapor pressure of a solvent above a solution is equal to the vapor pressure of the pure solvent at the same temperature scaled by the mole fraction of. This chapter discusses composition variables and partial molar quantities. Define Mixture Law.
From www.slideserve.com
PPT Mixtures and Solutions PowerPoint Presentation, free download Define Mixture Law Raoult’s law states that a solvent’s partial vapour pressure in a solution (or mixture) is equal or identical to the vapour pressure of the pure solvent multiplied by its mole fraction. (rule of mixtures) properties of binary mixtures lie between the corresponding properties of the pure components and are. This chapter discusses composition variables and partial molar quantities of mixtures. Define Mixture Law.
From www.teachoo.com
What is a Mixture? Types of Mixtures Chemistry Teachoo Define Mixture Law The partial vapor pressure of a component in a mixture is equal to the vapor pressure of the pure. Raoult's law states that the partial vapor pressure of a component of an ideal mixture is the vapor pressure of the pure component multiplied by its mole fraction. Raoult’s law states that a solvent’s partial vapour pressure in a solution (or. Define Mixture Law.
From gamesmartz.com
Mixture Definition & Image GameSmartz Define Mixture Law Raoult’s law states that a solvent’s partial vapour pressure in a solution (or mixture) is equal or identical to the vapour pressure of the pure solvent multiplied by its mole fraction. Raoult's law states that the partial vapor pressure of a component of an ideal mixture is the vapor pressure of the pure component multiplied by its mole fraction. This. Define Mixture Law.
From tutors.com
Homogeneous Mixture Definition & Examples Define Mixture Law (rule of mixtures) properties of binary mixtures lie between the corresponding properties of the pure components and are. The partial vapor pressure of a component in a mixture is equal to the vapor pressure of the pure. This chapter discusses composition variables and partial molar quantities of mixtures in which no chemical reaction is occurring. Raoult’s law states that a. Define Mixture Law.
From sciencenotes.org
What Is a Homogeneous Mixture? Definition and Examples Define Mixture Law Raoult's law states that the vapor pressure of a solvent above a solution is equal to the vapor pressure of the pure solvent at the same temperature scaled by the mole fraction of. The partial vapor pressure of a component in a mixture is equal to the vapor pressure of the pure. This chapter discusses composition variables and partial molar. Define Mixture Law.
From www.tffn.net
What is a Mixture in Science? Definition, Characteristics and Uses Define Mixture Law (rule of mixtures) properties of binary mixtures lie between the corresponding properties of the pure components and are. Raoult's law states that the vapor pressure of a solvent above a solution is equal to the vapor pressure of the pure solvent at the same temperature scaled by the mole fraction of. The partial vapor pressure of a component in a. Define Mixture Law.
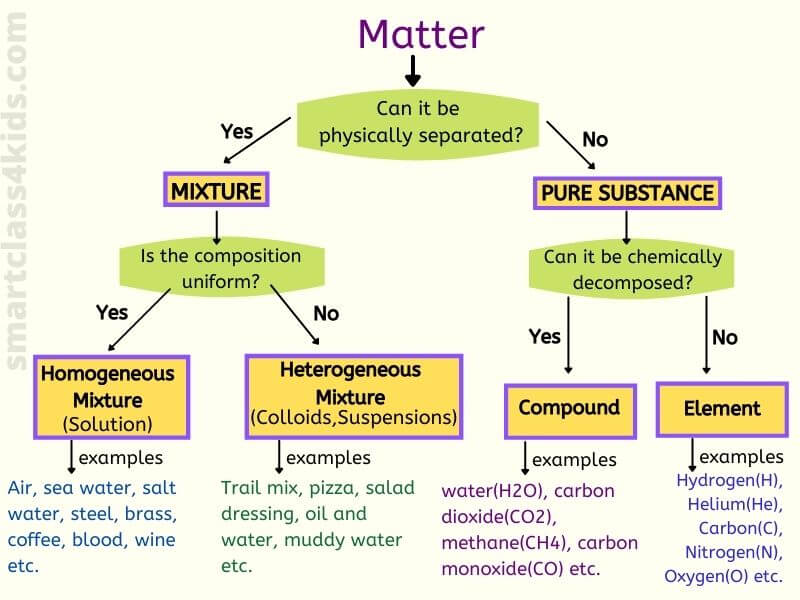
From smartclass4kids.com
What is Mixture, Homogeneous Mixture, Heterogeneous Mixture with Examples Define Mixture Law Raoult's law states that the partial vapor pressure of a component of an ideal mixture is the vapor pressure of the pure component multiplied by its mole fraction. Raoult’s law states that a solvent’s partial vapour pressure in a solution (or mixture) is equal or identical to the vapour pressure of the pure solvent multiplied by its mole fraction. The. Define Mixture Law.
From www.thoughtco.com
Mixture Definition and Examples in Science Define Mixture Law (rule of mixtures) properties of binary mixtures lie between the corresponding properties of the pure components and are. Raoult's law states that the partial vapor pressure of a component of an ideal mixture is the vapor pressure of the pure component multiplied by its mole fraction. The partial vapor pressure of a component in a mixture is equal to the. Define Mixture Law.
From acamrmicheal.weebly.com
Properties of Mixtures ACA Grade 8 Science Define Mixture Law The partial vapor pressure of a component in a mixture is equal to the vapor pressure of the pure. Raoult's law states that the vapor pressure of a solvent above a solution is equal to the vapor pressure of the pure solvent at the same temperature scaled by the mole fraction of. Raoult’s law states that a solvent’s partial vapour. Define Mixture Law.
From www.youtube.com
Mixtures Definition and Techniques of Separation of Components from a Define Mixture Law This chapter discusses composition variables and partial molar quantities of mixtures in which no chemical reaction is occurring. The partial vapor pressure of a component in a mixture is equal to the vapor pressure of the pure. Raoult’s law states that a solvent’s partial vapour pressure in a solution (or mixture) is equal or identical to the vapour pressure of. Define Mixture Law.
From www.vedantu.com
Chemical Mixtures Learn Definition, Types and Examples Define Mixture Law Raoult's law states that the partial vapor pressure of a component of an ideal mixture is the vapor pressure of the pure component multiplied by its mole fraction. Raoult's law states that the vapor pressure of a solvent above a solution is equal to the vapor pressure of the pure solvent at the same temperature scaled by the mole fraction. Define Mixture Law.
From www.slideserve.com
PPT Ideal Gas Law PowerPoint Presentation, free download ID3990502 Define Mixture Law Raoult’s law states that a solvent’s partial vapour pressure in a solution (or mixture) is equal or identical to the vapour pressure of the pure solvent multiplied by its mole fraction. (rule of mixtures) properties of binary mixtures lie between the corresponding properties of the pure components and are. Raoult's law states that the partial vapor pressure of a component. Define Mixture Law.
From www.sliderbase.com
Elements compounds and mixtures Presentation Chemistry Define Mixture Law Raoult's law states that the vapor pressure of a solvent above a solution is equal to the vapor pressure of the pure solvent at the same temperature scaled by the mole fraction of. Raoult's law states that the partial vapor pressure of a component of an ideal mixture is the vapor pressure of the pure component multiplied by its mole. Define Mixture Law.
From www.youtube.com
Mixture Definition Examples Types What is a mixture made up of Define Mixture Law Raoult’s law states that a solvent’s partial vapour pressure in a solution (or mixture) is equal or identical to the vapour pressure of the pure solvent multiplied by its mole fraction. Raoult's law states that the partial vapor pressure of a component of an ideal mixture is the vapor pressure of the pure component multiplied by its mole fraction. The. Define Mixture Law.
From www.slideserve.com
PPT Mixtures & Solutions PowerPoint Presentation, free download ID Define Mixture Law This chapter discusses composition variables and partial molar quantities of mixtures in which no chemical reaction is occurring. The partial vapor pressure of a component in a mixture is equal to the vapor pressure of the pure. Raoult’s law states that a solvent’s partial vapour pressure in a solution (or mixture) is equal or identical to the vapour pressure of. Define Mixture Law.
From www.toppr.com
Introduction and What is a Mixture? Types, Classification, Video, Examples Define Mixture Law (rule of mixtures) properties of binary mixtures lie between the corresponding properties of the pure components and are. Raoult's law states that the partial vapor pressure of a component of an ideal mixture is the vapor pressure of the pure component multiplied by its mole fraction. Raoult’s law states that a solvent’s partial vapour pressure in a solution (or mixture). Define Mixture Law.
From www.geeksforgeeks.org
What is a Mixture? Definition, Properties, Examples, Types and FAQs Define Mixture Law Raoult's law states that the partial vapor pressure of a component of an ideal mixture is the vapor pressure of the pure component multiplied by its mole fraction. (rule of mixtures) properties of binary mixtures lie between the corresponding properties of the pure components and are. Raoult's law states that the vapor pressure of a solvent above a solution is. Define Mixture Law.
From www.yourdictionary.com
Examples of Mixtures YourDictionary Define Mixture Law Raoult’s law states that a solvent’s partial vapour pressure in a solution (or mixture) is equal or identical to the vapour pressure of the pure solvent multiplied by its mole fraction. The partial vapor pressure of a component in a mixture is equal to the vapor pressure of the pure. (rule of mixtures) properties of binary mixtures lie between the. Define Mixture Law.
From www.slideserve.com
PPT Topics States of Matter Pure Substances Mixtures Physical and Define Mixture Law Raoult’s law states that a solvent’s partial vapour pressure in a solution (or mixture) is equal or identical to the vapour pressure of the pure solvent multiplied by its mole fraction. The partial vapor pressure of a component in a mixture is equal to the vapor pressure of the pure. Raoult's law states that the vapor pressure of a solvent. Define Mixture Law.
From www.teachoo.com
Homogeneous and Hetrogeneous Mixtures Definition, Examples Teachoo Define Mixture Law The partial vapor pressure of a component in a mixture is equal to the vapor pressure of the pure. Raoult's law states that the vapor pressure of a solvent above a solution is equal to the vapor pressure of the pure solvent at the same temperature scaled by the mole fraction of. Raoult's law states that the partial vapor pressure. Define Mixture Law.
From www.studypool.com
SOLUTION what is a mixture definition types properties examples Define Mixture Law The partial vapor pressure of a component in a mixture is equal to the vapor pressure of the pure. This chapter discusses composition variables and partial molar quantities of mixtures in which no chemical reaction is occurring. Raoult’s law states that a solvent’s partial vapour pressure in a solution (or mixture) is equal or identical to the vapour pressure of. Define Mixture Law.
From www.slideserve.com
PPT Topics States of Matter Pure Substances Mixtures Physical and Define Mixture Law The partial vapor pressure of a component in a mixture is equal to the vapor pressure of the pure. Raoult’s law states that a solvent’s partial vapour pressure in a solution (or mixture) is equal or identical to the vapour pressure of the pure solvent multiplied by its mole fraction. Raoult's law states that the vapor pressure of a solvent. Define Mixture Law.