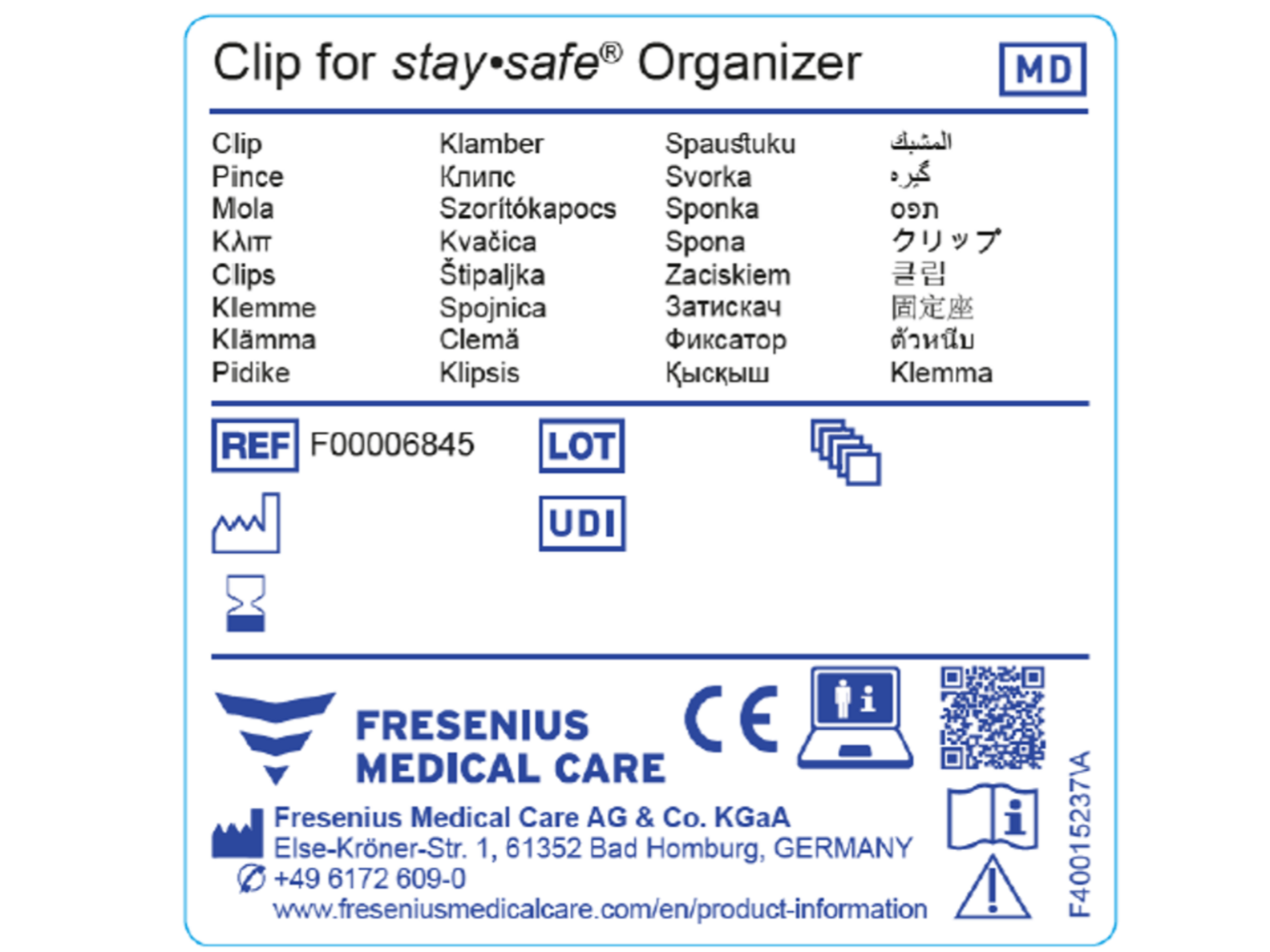
Medical Device Label Changes . Overview of regulations for medical devices: Legislative changes in the medical devices industry mean manufacturers may need to revisit their products, processes, and packaging to meet new. Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be brought to the. To help understand the different types of changes, we will cover four different areas for changes related to medical devices;. Manufacturers are required to submit a new 510(k) when a change (or changes) exceeds the 21 cfr 807.81(a)(3) threshold, “could significantly. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). The symbol was developed specifically to meet the gspr 23.2 (q) “an indication that the.
from www.freseniusmedicalcare.com
Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be brought to the. Legislative changes in the medical devices industry mean manufacturers may need to revisit their products, processes, and packaging to meet new. Overview of regulations for medical devices: The symbol was developed specifically to meet the gspr 23.2 (q) “an indication that the. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). To help understand the different types of changes, we will cover four different areas for changes related to medical devices;. Manufacturers are required to submit a new 510(k) when a change (or changes) exceeds the 21 cfr 807.81(a)(3) threshold, “could significantly.
Medical device regulation Fresenius Medical Care
Medical Device Label Changes The symbol was developed specifically to meet the gspr 23.2 (q) “an indication that the. The symbol was developed specifically to meet the gspr 23.2 (q) “an indication that the. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). To help understand the different types of changes, we will cover four different areas for changes related to medical devices;. Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be brought to the. Legislative changes in the medical devices industry mean manufacturers may need to revisit their products, processes, and packaging to meet new. Overview of regulations for medical devices: Manufacturers are required to submit a new 510(k) when a change (or changes) exceeds the 21 cfr 807.81(a)(3) threshold, “could significantly.
From old.sermitsiaq.ag
Medical Device Label Template Medical Device Label Changes To help understand the different types of changes, we will cover four different areas for changes related to medical devices;. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). The symbol was developed specifically to meet the gspr 23.2 (q) “an indication that the. Overview of regulations. Medical Device Label Changes.
From gbu-taganskij.ru
EU MDR Medical Device Labeling Changes And Challenges By, 47 OFF Medical Device Label Changes Legislative changes in the medical devices industry mean manufacturers may need to revisit their products, processes, and packaging to meet new. The symbol was developed specifically to meet the gspr 23.2 (q) “an indication that the. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Whenever the. Medical Device Label Changes.
From clin-r.com
Labels for Medical Devices Clin R Medical Device Label Changes Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be brought to the. The symbol was developed specifically to meet the gspr 23.2 (q) “an indication that the. Legislative changes in the medical devices industry mean manufacturers may need to revisit their products, processes, and packaging to meet. Medical Device Label Changes.
From www.regdesk.co
HSA Guidance on Labeling for Medical Devices Introduction RegDesk Medical Device Label Changes Legislative changes in the medical devices industry mean manufacturers may need to revisit their products, processes, and packaging to meet new. Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be brought to the. Manufacturers are required to submit a new 510(k) when a change (or changes) exceeds. Medical Device Label Changes.
From vascufirst.com
What is the meaning of symbols on medical devices labels? VascuFirst Medical Device Label Changes The symbol was developed specifically to meet the gspr 23.2 (q) “an indication that the. Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be brought to the. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal. Medical Device Label Changes.
From peakvascularaccess.com
What is the meaning of symbols on medical devices labels? Peak Mobile Medical Device Label Changes Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be brought to the. Legislative changes in the medical devices industry mean manufacturers may need to revisit their products, processes, and packaging to meet new. Labeling regulations pertaining to medical devices are found in the following parts of title. Medical Device Label Changes.
From www.afpharmaservice.com
Medical Device Labelling Requirements Medical Device Label Changes Overview of regulations for medical devices: To help understand the different types of changes, we will cover four different areas for changes related to medical devices;. Legislative changes in the medical devices industry mean manufacturers may need to revisit their products, processes, and packaging to meet new. Whenever the label of a medical device includes a printed expiration date, date. Medical Device Label Changes.
From www.techsollifesciences.com
EU MDR & IVDR Medical Device Labelling Requirements Medical Device Label Changes Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Legislative changes in the medical devices industry mean manufacturers may need to revisit their products, processes, and packaging to meet new. Overview of regulations for medical devices: The symbol was developed specifically to meet the gspr 23.2 (q). Medical Device Label Changes.
From www.regdesk.co
FDA Guidance on General Device Labeling RegDesk Medical Device Label Changes To help understand the different types of changes, we will cover four different areas for changes related to medical devices;. Overview of regulations for medical devices: The symbol was developed specifically to meet the gspr 23.2 (q) “an indication that the. Legislative changes in the medical devices industry mean manufacturers may need to revisit their products, processes, and packaging to. Medical Device Label Changes.
From www.meddeviceonline.com
Infographic Medical Device Label Before And After EU MDR 10 Sticking Points Medical Device Label Changes Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be brought to the. Legislative changes in the medical devices industry mean manufacturers may need to revisit their products, processes, and packaging to meet new. The symbol was developed specifically to meet the gspr 23.2 (q) “an indication that. Medical Device Label Changes.
From issuu.com
EU MDR Medical Device Labeling changes and challenges by martinafrotz Medical Device Label Changes To help understand the different types of changes, we will cover four different areas for changes related to medical devices;. The symbol was developed specifically to meet the gspr 23.2 (q) “an indication that the. Manufacturers are required to submit a new 510(k) when a change (or changes) exceeds the 21 cfr 807.81(a)(3) threshold, “could significantly. Labeling regulations pertaining to. Medical Device Label Changes.
From www.flexo-graphics.com
Medical Device Labeling Medical Equipment Labels Medical Device Label Changes Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be brought to the. Legislative changes in the medical devices industry mean manufacturers may need to revisit. Medical Device Label Changes.
From dandelionsandthings.blogspot.com
30 Medical Device Label Symbols Label Design Ideas 2020 Medical Device Label Changes Legislative changes in the medical devices industry mean manufacturers may need to revisit their products, processes, and packaging to meet new. Manufacturers are required to submit a new 510(k) when a change (or changes) exceeds the 21 cfr 807.81(a)(3) threshold, “could significantly. To help understand the different types of changes, we will cover four different areas for changes related to. Medical Device Label Changes.
From ambitiousmares.blogspot.com
35 Medical Device Label Labels Design Ideas 2020 Medical Device Label Changes Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be brought to the. The symbol was developed specifically to meet the gspr 23.2 (q) “an indication that the. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal. Medical Device Label Changes.
From peakvascularaccess.com
What is the meaning of symbols on medical devices labels? Peak Mobile Medical Device Label Changes Legislative changes in the medical devices industry mean manufacturers may need to revisit their products, processes, and packaging to meet new. Overview of regulations for medical devices: Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be brought to the. Labeling regulations pertaining to medical devices are found. Medical Device Label Changes.
From gbu-taganskij.ru
EU MDR Medical Device Labeling Changes And Challenges By, 47 OFF Medical Device Label Changes Legislative changes in the medical devices industry mean manufacturers may need to revisit their products, processes, and packaging to meet new. Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be brought to the. Manufacturers are required to submit a new 510(k) when a change (or changes) exceeds. Medical Device Label Changes.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Medical Device Label Changes Overview of regulations for medical devices: The symbol was developed specifically to meet the gspr 23.2 (q) “an indication that the. Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be brought to the. Labeling regulations pertaining to medical devices are found in the following parts of title. Medical Device Label Changes.
From www.slideserve.com
PPT Medical Device Labeling Regulation Changes by FDA for COVID 19 Medical Device Label Changes Overview of regulations for medical devices: Legislative changes in the medical devices industry mean manufacturers may need to revisit their products, processes, and packaging to meet new. Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be brought to the. The symbol was developed specifically to meet the. Medical Device Label Changes.
From www.freseniusmedicalcare.com
Medical device regulation Fresenius Medical Care Medical Device Label Changes Overview of regulations for medical devices: Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be brought to the. Legislative changes in the medical devices industry mean manufacturers may need to revisit their products, processes, and packaging to meet new. Labeling regulations pertaining to medical devices are found. Medical Device Label Changes.
From gbu-taganskij.ru
EU MDR 2017/745 Medical Device Labeling Compliance, 48 OFF Medical Device Label Changes Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). To help understand the different types of changes, we will cover four different areas for changes related to medical devices;. Legislative changes in the medical devices industry mean manufacturers may need to revisit their products, processes, and packaging. Medical Device Label Changes.
From peakvascularaccess.com
What is the meaning of symbols on medical devices labels? Peak Mobile Medical Device Label Changes To help understand the different types of changes, we will cover four different areas for changes related to medical devices;. The symbol was developed specifically to meet the gspr 23.2 (q) “an indication that the. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Manufacturers are required. Medical Device Label Changes.
From medenvoyglobal.com
Medical Device Labeling Requirements in Europe MedEnvoy Medical Device Label Changes Legislative changes in the medical devices industry mean manufacturers may need to revisit their products, processes, and packaging to meet new. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any. Medical Device Label Changes.
From exogphupj.blob.core.windows.net
Medical Device Labelling Tga at William Maurer blog Medical Device Label Changes To help understand the different types of changes, we will cover four different areas for changes related to medical devices;. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other. Medical Device Label Changes.
From gbu-taganskij.ru
EU MDR 2017/745 Medical Device Labeling Compliance, 48 OFF Medical Device Label Changes To help understand the different types of changes, we will cover four different areas for changes related to medical devices;. The symbol was developed specifically to meet the gspr 23.2 (q) “an indication that the. Legislative changes in the medical devices industry mean manufacturers may need to revisit their products, processes, and packaging to meet new. Whenever the label of. Medical Device Label Changes.
From vivafda.com
FDA Medical Device Labeling Requirements Viva FDA U.S. FDA Medical Device Label Changes Overview of regulations for medical devices: Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Legislative changes in the medical devices industry mean manufacturers may need to revisit their products, processes, and packaging to meet new. To help understand the different types of changes, we will cover. Medical Device Label Changes.
From medicaldevicelicense.com
Essential Medical Device Symbols for Labeling ISO 152231 Medical Device Label Changes Manufacturers are required to submit a new 510(k) when a change (or changes) exceeds the 21 cfr 807.81(a)(3) threshold, “could significantly. Overview of regulations for medical devices: Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). The symbol was developed specifically to meet the gspr 23.2 (q). Medical Device Label Changes.
From www.slideserve.com
PPT medical device labeling regulation Changes by FDA for covid 19 Medical Device Label Changes Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). The symbol was developed specifically to meet the gspr 23.2 (q) “an indication that the. Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be brought. Medical Device Label Changes.
From www.regdesk.co
FDA Guidance on Development of Medical Device Labeling RegDesk Medical Device Label Changes The symbol was developed specifically to meet the gspr 23.2 (q) “an indication that the. Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be brought to the. Legislative changes in the medical devices industry mean manufacturers may need to revisit their products, processes, and packaging to meet. Medical Device Label Changes.
From www.regdesk.co
FDA on General Principles of Labeling for Medical Devices RegDesk Medical Device Label Changes Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). The symbol was developed specifically to meet the gspr 23.2 (q) “an indication that the. Legislative changes in the medical devices industry mean manufacturers may need to revisit their products, processes, and packaging to meet new. Whenever the. Medical Device Label Changes.
From www.slideserve.com
PPT Medical Device Labeling PowerPoint Presentation, free download Medical Device Label Changes Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be brought to the. The symbol was developed specifically to meet the gspr 23.2 (q) “an indication that the. To help understand the different types of changes, we will cover four different areas for changes related to medical devices;.. Medical Device Label Changes.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Medical Device Label Changes Overview of regulations for medical devices: The symbol was developed specifically to meet the gspr 23.2 (q) “an indication that the. Manufacturers are required to submit a new 510(k) when a change (or changes) exceeds the 21 cfr 807.81(a)(3) threshold, “could significantly. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code. Medical Device Label Changes.
From www.celegence.com
Medical Device Labeling Changes and Challenges Under EU MDR Medical Device Label Changes Overview of regulations for medical devices: Manufacturers are required to submit a new 510(k) when a change (or changes) exceeds the 21 cfr 807.81(a)(3) threshold, “could significantly. The symbol was developed specifically to meet the gspr 23.2 (q) “an indication that the. To help understand the different types of changes, we will cover four different areas for changes related to. Medical Device Label Changes.
From clin-r.com
Labels for Medical Devices Clin R Medical Device Label Changes Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be brought to the. Overview of regulations for medical devices: Manufacturers are required to submit a new 510(k) when a change (or changes) exceeds the 21 cfr 807.81(a)(3) threshold, “could significantly. Labeling regulations pertaining to medical devices are found. Medical Device Label Changes.
From www.slideserve.com
PPT Medical Device Labeling PowerPoint Presentation, free download Medical Device Label Changes Manufacturers are required to submit a new 510(k) when a change (or changes) exceeds the 21 cfr 807.81(a)(3) threshold, “could significantly. The symbol was developed specifically to meet the gspr 23.2 (q) “an indication that the. To help understand the different types of changes, we will cover four different areas for changes related to medical devices;. Legislative changes in the. Medical Device Label Changes.
From coastlabel.com
Medical Device Labeling Medical Equipment Labels Coast Label Medical Device Label Changes Manufacturers are required to submit a new 510(k) when a change (or changes) exceeds the 21 cfr 807.81(a)(3) threshold, “could significantly. Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be brought to the. Overview of regulations for medical devices: To help understand the different types of changes,. Medical Device Label Changes.