What Is The Energy Balance To Open System . the energy balance equation for the open system is thus summation of energy balance equation for the closed system and energy. energy (as internal and flow energy) enters the system with the fluid flowing into the heater. identify relevant terms for energy balances for open and closed systems. A system is open if mass crosses the system boundary. Δh = 0 if there are no temperature changes, phase changes, or. Heat is also supplied to the. open system energy balance equation: For such a system, work must. energy balances on open systems. in this chapter we will introduce some of the basic properties required to perform energy balances on a. A closed system is one in which there is a fixed volume or space and no streams entering or leaving the. identify relevant terms for energy balances for open and closed systems. Use thermodynamic data tables to identify. Use thermodynamic data tables to identify enthalpy,.
from www.slideserve.com
open system energy balance equation: in this chapter we will introduce some of the basic properties required to perform energy balances on a. For such a system, work must. Use thermodynamic data tables to identify enthalpy,. energy (as internal and flow energy) enters the system with the fluid flowing into the heater. Use thermodynamic data tables to identify. Δh = 0 if there are no temperature changes, phase changes, or. the energy balance equation for the open system is thus summation of energy balance equation for the closed system and energy. energy balances on open systems. Heat is also supplied to the.
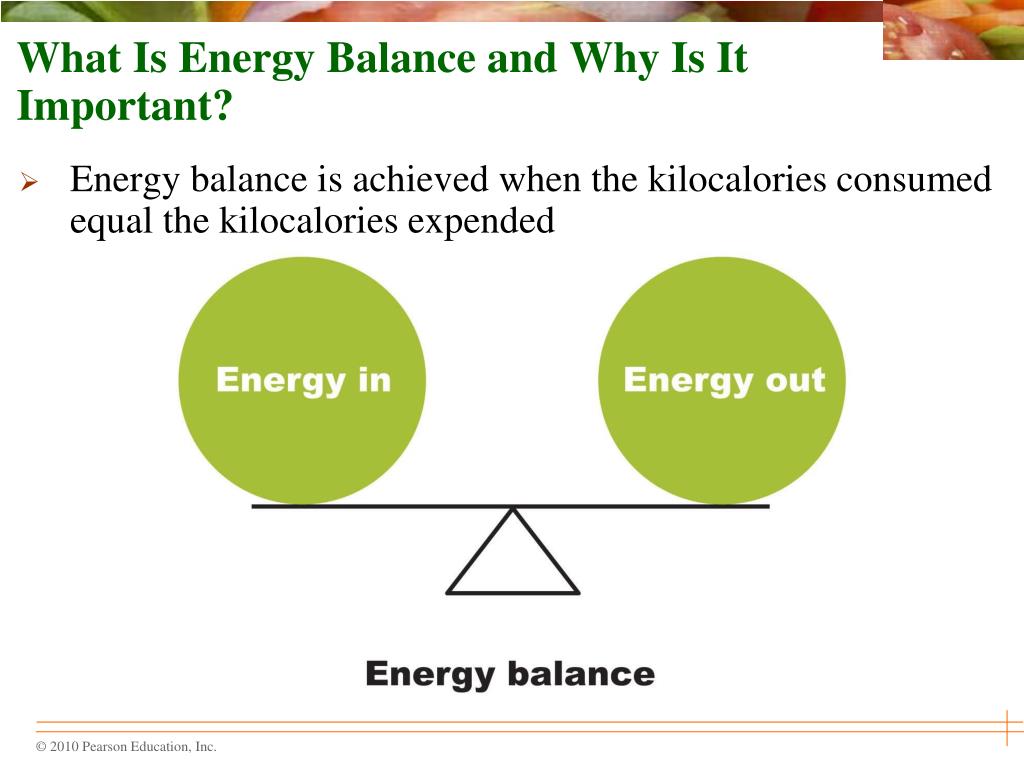
PPT Chapter 14 Energy Balance and Body Composition PowerPoint
What Is The Energy Balance To Open System the energy balance equation for the open system is thus summation of energy balance equation for the closed system and energy. For such a system, work must. energy balances on open systems. identify relevant terms for energy balances for open and closed systems. energy (as internal and flow energy) enters the system with the fluid flowing into the heater. Δh = 0 if there are no temperature changes, phase changes, or. open system energy balance equation: Use thermodynamic data tables to identify enthalpy,. identify relevant terms for energy balances for open and closed systems. in this chapter we will introduce some of the basic properties required to perform energy balances on a. Use thermodynamic data tables to identify. A closed system is one in which there is a fixed volume or space and no streams entering or leaving the. A system is open if mass crosses the system boundary. Heat is also supplied to the. the energy balance equation for the open system is thus summation of energy balance equation for the closed system and energy.
From www.youtube.com
Chapter 7.4 Ex2 Energy Balance for An Open System (Principle of What Is The Energy Balance To Open System Use thermodynamic data tables to identify enthalpy,. Use thermodynamic data tables to identify. A closed system is one in which there is a fixed volume or space and no streams entering or leaving the. Δh = 0 if there are no temperature changes, phase changes, or. For such a system, work must. identify relevant terms for energy balances for. What Is The Energy Balance To Open System.
From www.studocu.com
Module 4. Energy Balance Open System Chemical Engineering What Is The Energy Balance To Open System energy balances on open systems. For such a system, work must. Heat is also supplied to the. energy (as internal and flow energy) enters the system with the fluid flowing into the heater. in this chapter we will introduce some of the basic properties required to perform energy balances on a. Δh = 0 if there are. What Is The Energy Balance To Open System.
From www.youtube.com
Thermodynamics Lecture 12 Control Volume Energy Balance YouTube What Is The Energy Balance To Open System A system is open if mass crosses the system boundary. in this chapter we will introduce some of the basic properties required to perform energy balances on a. the energy balance equation for the open system is thus summation of energy balance equation for the closed system and energy. identify relevant terms for energy balances for open. What Is The Energy Balance To Open System.
From www.youtube.com
Open and Closed Systems YouTube What Is The Energy Balance To Open System the energy balance equation for the open system is thus summation of energy balance equation for the closed system and energy. open system energy balance equation: Heat is also supplied to the. in this chapter we will introduce some of the basic properties required to perform energy balances on a. Use thermodynamic data tables to identify. . What Is The Energy Balance To Open System.
From www.youtube.com
Energy Balance of an Open System YouTube What Is The Energy Balance To Open System Use thermodynamic data tables to identify enthalpy,. A closed system is one in which there is a fixed volume or space and no streams entering or leaving the. the energy balance equation for the open system is thus summation of energy balance equation for the closed system and energy. Use thermodynamic data tables to identify. identify relevant terms. What Is The Energy Balance To Open System.
From www.slideserve.com
PPT Lecture 19 PowerPoint Presentation, free download ID2507242 What Is The Energy Balance To Open System A closed system is one in which there is a fixed volume or space and no streams entering or leaving the. open system energy balance equation: For such a system, work must. in this chapter we will introduce some of the basic properties required to perform energy balances on a. identify relevant terms for energy balances for. What Is The Energy Balance To Open System.
From www.slideserve.com
PPT Chapter 14 Energy Balance and Body Composition PowerPoint What Is The Energy Balance To Open System A system is open if mass crosses the system boundary. identify relevant terms for energy balances for open and closed systems. Heat is also supplied to the. energy (as internal and flow energy) enters the system with the fluid flowing into the heater. open system energy balance equation: energy balances on open systems. Use thermodynamic data. What Is The Energy Balance To Open System.
From www.slideserve.com
PPT Lecture 5 Energy Balance PowerPoint Presentation, free download What Is The Energy Balance To Open System in this chapter we will introduce some of the basic properties required to perform energy balances on a. Heat is also supplied to the. For such a system, work must. open system energy balance equation: Δh = 0 if there are no temperature changes, phase changes, or. A closed system is one in which there is a fixed. What Is The Energy Balance To Open System.
From www.youtube.com
Simultaneous material and Energy balance for open system (steadystate What Is The Energy Balance To Open System For such a system, work must. identify relevant terms for energy balances for open and closed systems. identify relevant terms for energy balances for open and closed systems. Heat is also supplied to the. in this chapter we will introduce some of the basic properties required to perform energy balances on a. Δh = 0 if there. What Is The Energy Balance To Open System.
From www.mdanderson.org
Energy balance What is it, and how can you achieve it? MD Anderson What Is The Energy Balance To Open System the energy balance equation for the open system is thus summation of energy balance equation for the closed system and energy. Heat is also supplied to the. For such a system, work must. Δh = 0 if there are no temperature changes, phase changes, or. Use thermodynamic data tables to identify. open system energy balance equation: identify. What Is The Energy Balance To Open System.
From www.youtube.com
Lec 73 OpenSystems Energy Balance, Reference States & State What Is The Energy Balance To Open System Use thermodynamic data tables to identify. the energy balance equation for the open system is thus summation of energy balance equation for the closed system and energy. Δh = 0 if there are no temperature changes, phase changes, or. A closed system is one in which there is a fixed volume or space and no streams entering or leaving. What Is The Energy Balance To Open System.
From www.slideserve.com
PPT Energy Balances on Open Systems at SteadyState PowerPoint What Is The Energy Balance To Open System A system is open if mass crosses the system boundary. in this chapter we will introduce some of the basic properties required to perform energy balances on a. energy balances on open systems. Use thermodynamic data tables to identify. energy (as internal and flow energy) enters the system with the fluid flowing into the heater. For such. What Is The Energy Balance To Open System.
From www.slideserve.com
PPT Energy Balance Equation PowerPoint Presentation, free download What Is The Energy Balance To Open System Use thermodynamic data tables to identify. in this chapter we will introduce some of the basic properties required to perform energy balances on a. open system energy balance equation: the energy balance equation for the open system is thus summation of energy balance equation for the closed system and energy. identify relevant terms for energy balances. What Is The Energy Balance To Open System.
From www.dreamstime.com
Energy Balance with Calories Intake and daily Expenditure Outline What Is The Energy Balance To Open System energy (as internal and flow energy) enters the system with the fluid flowing into the heater. energy balances on open systems. identify relevant terms for energy balances for open and closed systems. Use thermodynamic data tables to identify enthalpy,. Δh = 0 if there are no temperature changes, phase changes, or. identify relevant terms for energy. What Is The Energy Balance To Open System.
From www.youtube.com
Exergy Balance equation for Control Volume (open system) YouTube What Is The Energy Balance To Open System A system is open if mass crosses the system boundary. identify relevant terms for energy balances for open and closed systems. identify relevant terms for energy balances for open and closed systems. open system energy balance equation: For such a system, work must. Heat is also supplied to the. Use thermodynamic data tables to identify. in. What Is The Energy Balance To Open System.
From fyolhdrbt.blob.core.windows.net
Energy Balance Definition at William Atkinson blog What Is The Energy Balance To Open System identify relevant terms for energy balances for open and closed systems. A system is open if mass crosses the system boundary. Use thermodynamic data tables to identify. identify relevant terms for energy balances for open and closed systems. the energy balance equation for the open system is thus summation of energy balance equation for the closed system. What Is The Energy Balance To Open System.
From www.slideserve.com
PPT ENERGY BALANCE PowerPoint Presentation, free download ID1402513 What Is The Energy Balance To Open System For such a system, work must. Δh = 0 if there are no temperature changes, phase changes, or. identify relevant terms for energy balances for open and closed systems. Heat is also supplied to the. the energy balance equation for the open system is thus summation of energy balance equation for the closed system and energy. A system. What Is The Energy Balance To Open System.
From www.slideserve.com
PPT Chapter 14 Energy Balance and Body Composition PowerPoint What Is The Energy Balance To Open System the energy balance equation for the open system is thus summation of energy balance equation for the closed system and energy. Use thermodynamic data tables to identify enthalpy,. energy balances on open systems. A closed system is one in which there is a fixed volume or space and no streams entering or leaving the. open system energy. What Is The Energy Balance To Open System.
From pharmaguides.in
Energy Balance Equation And Heat Transfer Process What Is The Energy Balance To Open System identify relevant terms for energy balances for open and closed systems. Use thermodynamic data tables to identify. A system is open if mass crosses the system boundary. energy (as internal and flow energy) enters the system with the fluid flowing into the heater. Δh = 0 if there are no temperature changes, phase changes, or. identify relevant. What Is The Energy Balance To Open System.
From www.youtube.com
Lec 72 Energy Balances on Closed & Open Systems YouTube What Is The Energy Balance To Open System For such a system, work must. open system energy balance equation: Use thermodynamic data tables to identify. A system is open if mass crosses the system boundary. Δh = 0 if there are no temperature changes, phase changes, or. energy balances on open systems. Use thermodynamic data tables to identify enthalpy,. A closed system is one in which. What Is The Energy Balance To Open System.
From www.slideserve.com
PPT Lecture 3 The first law of thermodynamics PowerPoint What Is The Energy Balance To Open System A system is open if mass crosses the system boundary. energy balances on open systems. identify relevant terms for energy balances for open and closed systems. the energy balance equation for the open system is thus summation of energy balance equation for the closed system and energy. Heat is also supplied to the. in this chapter. What Is The Energy Balance To Open System.
From www.mix-fit.net.au
Energy Homeostasis and Energy Balance Mixfit What Is The Energy Balance To Open System Δh = 0 if there are no temperature changes, phase changes, or. Use thermodynamic data tables to identify. energy (as internal and flow energy) enters the system with the fluid flowing into the heater. energy balances on open systems. identify relevant terms for energy balances for open and closed systems. Heat is also supplied to the. A. What Is The Energy Balance To Open System.
From www.slideserve.com
PPT Chapter 2. Fundamental Concepts in Understanding Bioenergy and What Is The Energy Balance To Open System energy (as internal and flow energy) enters the system with the fluid flowing into the heater. A system is open if mass crosses the system boundary. identify relevant terms for energy balances for open and closed systems. in this chapter we will introduce some of the basic properties required to perform energy balances on a. the. What Is The Energy Balance To Open System.
From www.slideserve.com
PPT Entropy balance for Open Systems PowerPoint Presentation, free What Is The Energy Balance To Open System energy balances on open systems. in this chapter we will introduce some of the basic properties required to perform energy balances on a. Use thermodynamic data tables to identify enthalpy,. open system energy balance equation: Δh = 0 if there are no temperature changes, phase changes, or. For such a system, work must. identify relevant terms. What Is The Energy Balance To Open System.
From www.youtube.com
Thermodynamics and Fluid Mechanics C3 L3 Energy Balance for What Is The Energy Balance To Open System identify relevant terms for energy balances for open and closed systems. Use thermodynamic data tables to identify. Use thermodynamic data tables to identify enthalpy,. Heat is also supplied to the. in this chapter we will introduce some of the basic properties required to perform energy balances on a. open system energy balance equation: A system is open. What Is The Energy Balance To Open System.
From www.slideserve.com
PPT Entropy balance for Open Systems PowerPoint Presentation, free What Is The Energy Balance To Open System Use thermodynamic data tables to identify enthalpy,. Use thermodynamic data tables to identify. identify relevant terms for energy balances for open and closed systems. identify relevant terms for energy balances for open and closed systems. the energy balance equation for the open system is thus summation of energy balance equation for the closed system and energy. . What Is The Energy Balance To Open System.
From studylib.net
FIRST LAW IN OPEN SYSTEMS Steady Flow Energy Equation What Is The Energy Balance To Open System in this chapter we will introduce some of the basic properties required to perform energy balances on a. open system energy balance equation: energy balances on open systems. Δh = 0 if there are no temperature changes, phase changes, or. Use thermodynamic data tables to identify enthalpy,. identify relevant terms for energy balances for open and. What Is The Energy Balance To Open System.
From www.bartleby.com
Think of an example of an open system, and outline some of the matter What Is The Energy Balance To Open System energy balances on open systems. Use thermodynamic data tables to identify enthalpy,. energy (as internal and flow energy) enters the system with the fluid flowing into the heater. the energy balance equation for the open system is thus summation of energy balance equation for the closed system and energy. A system is open if mass crosses the. What Is The Energy Balance To Open System.
From www.tec-science.com
Flow work in open systems tecscience What Is The Energy Balance To Open System identify relevant terms for energy balances for open and closed systems. Use thermodynamic data tables to identify. Use thermodynamic data tables to identify enthalpy,. For such a system, work must. A system is open if mass crosses the system boundary. A closed system is one in which there is a fixed volume or space and no streams entering or. What Is The Energy Balance To Open System.
From www.youtube.com
Thermodynamics Chapter 3 Energy Balance Equation S YouTube What Is The Energy Balance To Open System Δh = 0 if there are no temperature changes, phase changes, or. identify relevant terms for energy balances for open and closed systems. identify relevant terms for energy balances for open and closed systems. A system is open if mass crosses the system boundary. A closed system is one in which there is a fixed volume or space. What Is The Energy Balance To Open System.
From www.youtube.com
Thermodynamics 1 C2 L10 First law of thermodynamics Energy What Is The Energy Balance To Open System Heat is also supplied to the. energy balances on open systems. identify relevant terms for energy balances for open and closed systems. the energy balance equation for the open system is thus summation of energy balance equation for the closed system and energy. For such a system, work must. Δh = 0 if there are no temperature. What Is The Energy Balance To Open System.
From www.youtube.com
Open System Energy Balance YouTube What Is The Energy Balance To Open System Use thermodynamic data tables to identify. energy (as internal and flow energy) enters the system with the fluid flowing into the heater. Use thermodynamic data tables to identify enthalpy,. Δh = 0 if there are no temperature changes, phase changes, or. the energy balance equation for the open system is thus summation of energy balance equation for the. What Is The Energy Balance To Open System.
From www.slideserve.com
PPT Lecture 5 Energy Balance PowerPoint Presentation, free download What Is The Energy Balance To Open System A closed system is one in which there is a fixed volume or space and no streams entering or leaving the. identify relevant terms for energy balances for open and closed systems. For such a system, work must. the energy balance equation for the open system is thus summation of energy balance equation for the closed system and. What Is The Energy Balance To Open System.
From www.slideserve.com
PPT ENERGY BALANCE PowerPoint Presentation, free download ID2132590 What Is The Energy Balance To Open System Use thermodynamic data tables to identify enthalpy,. For such a system, work must. energy balances on open systems. the energy balance equation for the open system is thus summation of energy balance equation for the closed system and energy. identify relevant terms for energy balances for open and closed systems. A system is open if mass crosses. What Is The Energy Balance To Open System.
From www.youtube.com
1 PP 27 Energy in Open and Close Systems YouTube What Is The Energy Balance To Open System A closed system is one in which there is a fixed volume or space and no streams entering or leaving the. the energy balance equation for the open system is thus summation of energy balance equation for the closed system and energy. Δh = 0 if there are no temperature changes, phase changes, or. A system is open if. What Is The Energy Balance To Open System.