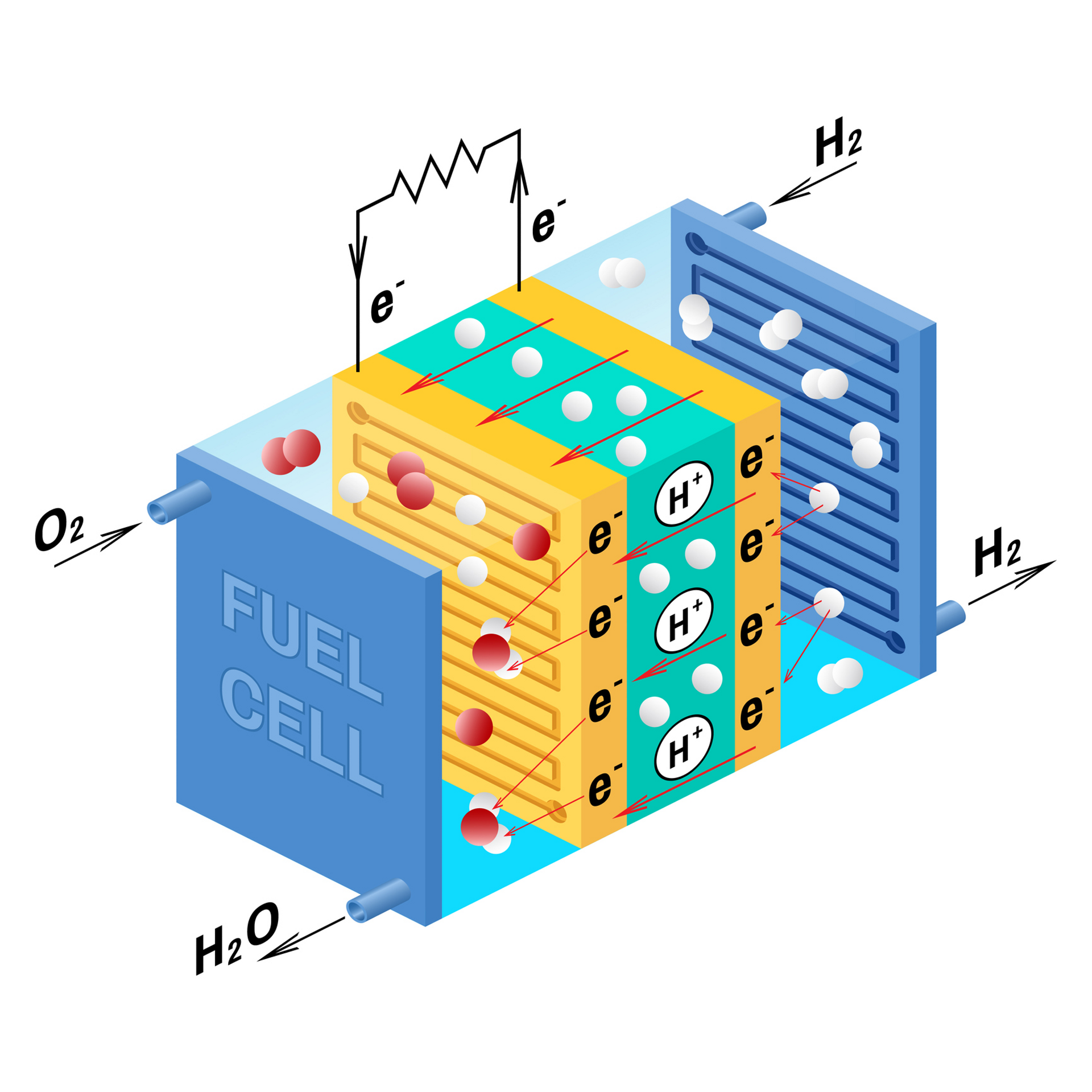
Fuel Cells Ncert . Electrochemistry notes class 12 covers a brief outline of topics such as electrochemical cells, nernst equation, gibbs. What is a fuel cell? A fuel cell is a device that produces electric energy, through a chemical reaction. It is defined as an electrochemical cell that generates electrical energy from fuel via electrochemical reactions. A fuel cell is a tiny device, capable of generating electricity by force of a chemical reaction. Learn types of fuel cell, working and more here. Fuel cells use a positively charged ion (hydrogen) and an oxidizing agent (oxygen). In the presence of two electrodes, a. A fuel cell can be described as an electrochemical cell which, through an electrochemical reaction, generates electrical energy from the fuel.
from www.linquip.com
Fuel cells use a positively charged ion (hydrogen) and an oxidizing agent (oxygen). It is defined as an electrochemical cell that generates electrical energy from fuel via electrochemical reactions. A fuel cell is a tiny device, capable of generating electricity by force of a chemical reaction. A fuel cell is a device that produces electric energy, through a chemical reaction. Electrochemistry notes class 12 covers a brief outline of topics such as electrochemical cells, nernst equation, gibbs. What is a fuel cell? Learn types of fuel cell, working and more here. In the presence of two electrodes, a. A fuel cell can be described as an electrochemical cell which, through an electrochemical reaction, generates electrical energy from the fuel.
Fuel Cells Advantages and Disadvantages in 2021 Linquip
Fuel Cells Ncert A fuel cell can be described as an electrochemical cell which, through an electrochemical reaction, generates electrical energy from the fuel. Learn types of fuel cell, working and more here. What is a fuel cell? In the presence of two electrodes, a. It is defined as an electrochemical cell that generates electrical energy from fuel via electrochemical reactions. A fuel cell is a device that produces electric energy, through a chemical reaction. A fuel cell can be described as an electrochemical cell which, through an electrochemical reaction, generates electrical energy from the fuel. A fuel cell is a tiny device, capable of generating electricity by force of a chemical reaction. Electrochemistry notes class 12 covers a brief outline of topics such as electrochemical cells, nernst equation, gibbs. Fuel cells use a positively charged ion (hydrogen) and an oxidizing agent (oxygen).
From www.fuelcellenergy.com
The Basics of FuelCell Energy’s Carbon Capture Platform Fuel Cells Ncert Learn types of fuel cell, working and more here. Electrochemistry notes class 12 covers a brief outline of topics such as electrochemical cells, nernst equation, gibbs. A fuel cell can be described as an electrochemical cell which, through an electrochemical reaction, generates electrical energy from the fuel. What is a fuel cell? Fuel cells use a positively charged ion (hydrogen). Fuel Cells Ncert.
From www.tes.com
Fuel Cells GCSE AQA Teaching Resources Fuel Cells Ncert A fuel cell can be described as an electrochemical cell which, through an electrochemical reaction, generates electrical energy from the fuel. A fuel cell is a device that produces electric energy, through a chemical reaction. It is defined as an electrochemical cell that generates electrical energy from fuel via electrochemical reactions. What is a fuel cell? Fuel cells use a. Fuel Cells Ncert.
From climatebiz.com
Hydrogen fuel cells vs. lithiumion batteries Powering EVs Fuel Cells Ncert Electrochemistry notes class 12 covers a brief outline of topics such as electrochemical cells, nernst equation, gibbs. What is a fuel cell? Fuel cells use a positively charged ion (hydrogen) and an oxidizing agent (oxygen). A fuel cell is a tiny device, capable of generating electricity by force of a chemical reaction. In the presence of two electrodes, a. It. Fuel Cells Ncert.
From www.youtube.com
GCSE Chemistry Fuel Cells 45 YouTube Fuel Cells Ncert It is defined as an electrochemical cell that generates electrical energy from fuel via electrochemical reactions. In the presence of two electrodes, a. Learn types of fuel cell, working and more here. Electrochemistry notes class 12 covers a brief outline of topics such as electrochemical cells, nernst equation, gibbs. What is a fuel cell? A fuel cell is a tiny. Fuel Cells Ncert.
From www.fuelcellenergy.com
Benefits of Fuel Cells in Energy Efficient Power Generation Fuel Cells Ncert Fuel cells use a positively charged ion (hydrogen) and an oxidizing agent (oxygen). It is defined as an electrochemical cell that generates electrical energy from fuel via electrochemical reactions. Electrochemistry notes class 12 covers a brief outline of topics such as electrochemical cells, nernst equation, gibbs. Learn types of fuel cell, working and more here. In the presence of two. Fuel Cells Ncert.
From www.youtube.com
Electrochemistry( Part 6) Batteries, Fuel Cells, Corrosion NCERT Fuel Cells Ncert Electrochemistry notes class 12 covers a brief outline of topics such as electrochemical cells, nernst equation, gibbs. It is defined as an electrochemical cell that generates electrical energy from fuel via electrochemical reactions. Fuel cells use a positively charged ion (hydrogen) and an oxidizing agent (oxygen). In the presence of two electrodes, a. What is a fuel cell? A fuel. Fuel Cells Ncert.
From www.chfca.ca
About Fuel Cells CHFCA Fuel Cells Ncert What is a fuel cell? Electrochemistry notes class 12 covers a brief outline of topics such as electrochemical cells, nernst equation, gibbs. In the presence of two electrodes, a. Learn types of fuel cell, working and more here. A fuel cell can be described as an electrochemical cell which, through an electrochemical reaction, generates electrical energy from the fuel. It. Fuel Cells Ncert.
From askfilo.com
FROM NCERT 凸 51 A fuel cell develops an electrical potential from the co.. Fuel Cells Ncert Electrochemistry notes class 12 covers a brief outline of topics such as electrochemical cells, nernst equation, gibbs. What is a fuel cell? A fuel cell is a tiny device, capable of generating electricity by force of a chemical reaction. Fuel cells use a positively charged ion (hydrogen) and an oxidizing agent (oxygen). It is defined as an electrochemical cell that. Fuel Cells Ncert.
From www.researchgate.net
Working principle of a hybrid fuel cell, composed of a high pH cathode Fuel Cells Ncert What is a fuel cell? Fuel cells use a positively charged ion (hydrogen) and an oxidizing agent (oxygen). In the presence of two electrodes, a. Electrochemistry notes class 12 covers a brief outline of topics such as electrochemical cells, nernst equation, gibbs. A fuel cell can be described as an electrochemical cell which, through an electrochemical reaction, generates electrical energy. Fuel Cells Ncert.
From www.youtube.com
08 Electrochemistry Fuel Cell and Corrosion For 12th NCERT IIT JEE NEET Fuel Cells Ncert Electrochemistry notes class 12 covers a brief outline of topics such as electrochemical cells, nernst equation, gibbs. In the presence of two electrodes, a. A fuel cell is a tiny device, capable of generating electricity by force of a chemical reaction. A fuel cell is a device that produces electric energy, through a chemical reaction. It is defined as an. Fuel Cells Ncert.
From www.youtube.com
Electrochemistry lec15Lead storage cellFuel cellJEENEETNCERT Fuel Cells Ncert Electrochemistry notes class 12 covers a brief outline of topics such as electrochemical cells, nernst equation, gibbs. Fuel cells use a positively charged ion (hydrogen) and an oxidizing agent (oxygen). Learn types of fuel cell, working and more here. In the presence of two electrodes, a. A fuel cell can be described as an electrochemical cell which, through an electrochemical. Fuel Cells Ncert.
From www.pnas.org
Carbonatesuperstructured solid fuel cells with hydrocarbon fuels PNAS Fuel Cells Ncert A fuel cell can be described as an electrochemical cell which, through an electrochemical reaction, generates electrical energy from the fuel. A fuel cell is a device that produces electric energy, through a chemical reaction. In the presence of two electrodes, a. Electrochemistry notes class 12 covers a brief outline of topics such as electrochemical cells, nernst equation, gibbs. What. Fuel Cells Ncert.
From www.plugpower.com
Fuel Cell Efficiency 101 The Key Metrics Plug Power Fuel Cells Ncert Electrochemistry notes class 12 covers a brief outline of topics such as electrochemical cells, nernst equation, gibbs. A fuel cell can be described as an electrochemical cell which, through an electrochemical reaction, generates electrical energy from the fuel. A fuel cell is a tiny device, capable of generating electricity by force of a chemical reaction. What is a fuel cell?. Fuel Cells Ncert.
From www.youtube.com
ईंधन सेल क्लास 12 chemistry। fuel cell NCERT / YouTube Fuel Cells Ncert A fuel cell can be described as an electrochemical cell which, through an electrochemical reaction, generates electrical energy from the fuel. It is defined as an electrochemical cell that generates electrical energy from fuel via electrochemical reactions. Electrochemistry notes class 12 covers a brief outline of topics such as electrochemical cells, nernst equation, gibbs. A fuel cell is a device. Fuel Cells Ncert.
From www.vedantu.com
Draw a neat labelled diagram of {H_2} {O_2} fuel cell. Write the Fuel Cells Ncert Electrochemistry notes class 12 covers a brief outline of topics such as electrochemical cells, nernst equation, gibbs. In the presence of two electrodes, a. A fuel cell can be described as an electrochemical cell which, through an electrochemical reaction, generates electrical energy from the fuel. Learn types of fuel cell, working and more here. Fuel cells use a positively charged. Fuel Cells Ncert.
From siqens.de
Methanol fuel cell Working principle and different types SIQENS Fuel Cells Ncert In the presence of two electrodes, a. Learn types of fuel cell, working and more here. Electrochemistry notes class 12 covers a brief outline of topics such as electrochemical cells, nernst equation, gibbs. A fuel cell is a device that produces electric energy, through a chemical reaction. A fuel cell is a tiny device, capable of generating electricity by force. Fuel Cells Ncert.
From www.youtube.com
Comparison of Fuel Cells YouTube Fuel Cells Ncert A fuel cell is a tiny device, capable of generating electricity by force of a chemical reaction. It is defined as an electrochemical cell that generates electrical energy from fuel via electrochemical reactions. A fuel cell is a device that produces electric energy, through a chemical reaction. In the presence of two electrodes, a. A fuel cell can be described. Fuel Cells Ncert.
From www.engineeringity.com
Breaking Down Fuel Cells How they Work and their Components The Fuel Cells Ncert A fuel cell is a device that produces electric energy, through a chemical reaction. A fuel cell is a tiny device, capable of generating electricity by force of a chemical reaction. It is defined as an electrochemical cell that generates electrical energy from fuel via electrochemical reactions. A fuel cell can be described as an electrochemical cell which, through an. Fuel Cells Ncert.
From www.youtube.com
3.17Fuel cell class 12th,Electrochemistry YouTube Fuel Cells Ncert Fuel cells use a positively charged ion (hydrogen) and an oxidizing agent (oxygen). What is a fuel cell? Learn types of fuel cell, working and more here. A fuel cell is a tiny device, capable of generating electricity by force of a chemical reaction. Electrochemistry notes class 12 covers a brief outline of topics such as electrochemical cells, nernst equation,. Fuel Cells Ncert.
From www.nextbigfuture.com
Molten Carbonate High Temperature Fuel Cells Getting to Scale Fuel Cells Ncert A fuel cell can be described as an electrochemical cell which, through an electrochemical reaction, generates electrical energy from the fuel. Learn types of fuel cell, working and more here. Fuel cells use a positively charged ion (hydrogen) and an oxidizing agent (oxygen). What is a fuel cell? Electrochemistry notes class 12 covers a brief outline of topics such as. Fuel Cells Ncert.
From faculty.fairfield.edu
Project 2 template Fuel Cells Ncert What is a fuel cell? Fuel cells use a positively charged ion (hydrogen) and an oxidizing agent (oxygen). A fuel cell is a device that produces electric energy, through a chemical reaction. Learn types of fuel cell, working and more here. Electrochemistry notes class 12 covers a brief outline of topics such as electrochemical cells, nernst equation, gibbs. It is. Fuel Cells Ncert.
From www.youtube.com
Electrochemistry 05 Kohlraush Law Batteries Fuel Cells Fuel Cells Ncert A fuel cell is a device that produces electric energy, through a chemical reaction. A fuel cell is a tiny device, capable of generating electricity by force of a chemical reaction. Learn types of fuel cell, working and more here. What is a fuel cell? It is defined as an electrochemical cell that generates electrical energy from fuel via electrochemical. Fuel Cells Ncert.
From www.doubtnut.com
Fuel Cells from NCERT FINGERTIPS CHEMISTRY (HINGLISH) ELECTROCHEMISTRY Fuel Cells Ncert Learn types of fuel cell, working and more here. A fuel cell is a device that produces electric energy, through a chemical reaction. Fuel cells use a positively charged ion (hydrogen) and an oxidizing agent (oxygen). In the presence of two electrodes, a. It is defined as an electrochemical cell that generates electrical energy from fuel via electrochemical reactions. A. Fuel Cells Ncert.
From www.youtube.com
Fuel Cells Advantages of Fuel Cells Class12 NCERT Chaper3 Fuel Cells Ncert A fuel cell is a device that produces electric energy, through a chemical reaction. Fuel cells use a positively charged ion (hydrogen) and an oxidizing agent (oxygen). Electrochemistry notes class 12 covers a brief outline of topics such as electrochemical cells, nernst equation, gibbs. What is a fuel cell? A fuel cell can be described as an electrochemical cell which,. Fuel Cells Ncert.
From www.linquip.com
Fuel Cells Advantages and Disadvantages in 2021 Linquip Fuel Cells Ncert A fuel cell can be described as an electrochemical cell which, through an electrochemical reaction, generates electrical energy from the fuel. Fuel cells use a positively charged ion (hydrogen) and an oxidizing agent (oxygen). Electrochemistry notes class 12 covers a brief outline of topics such as electrochemical cells, nernst equation, gibbs. What is a fuel cell? A fuel cell is. Fuel Cells Ncert.
From www.nagwa.com
Question Video Identifying Which Equation Shows the Reaction at a Fuel Cells Ncert A fuel cell is a device that produces electric energy, through a chemical reaction. A fuel cell can be described as an electrochemical cell which, through an electrochemical reaction, generates electrical energy from the fuel. Fuel cells use a positively charged ion (hydrogen) and an oxidizing agent (oxygen). In the presence of two electrodes, a. What is a fuel cell?. Fuel Cells Ncert.
From www.studypool.com
SOLUTION Fuel cells notes Studypool Fuel Cells Ncert Learn types of fuel cell, working and more here. Electrochemistry notes class 12 covers a brief outline of topics such as electrochemical cells, nernst equation, gibbs. A fuel cell is a tiny device, capable of generating electricity by force of a chemical reaction. In the presence of two electrodes, a. It is defined as an electrochemical cell that generates electrical. Fuel Cells Ncert.
From mungfali.com
Fuel Cell Schematic Fuel Cells Ncert Electrochemistry notes class 12 covers a brief outline of topics such as electrochemical cells, nernst equation, gibbs. A fuel cell is a tiny device, capable of generating electricity by force of a chemical reaction. A fuel cell is a device that produces electric energy, through a chemical reaction. In the presence of two electrodes, a. Fuel cells use a positively. Fuel Cells Ncert.
From cbselibrary.com
Fuel Cell Advantages And Disadvantages Application of Fuel Cell Fuel Cells Ncert What is a fuel cell? Electrochemistry notes class 12 covers a brief outline of topics such as electrochemical cells, nernst equation, gibbs. A fuel cell is a tiny device, capable of generating electricity by force of a chemical reaction. It is defined as an electrochemical cell that generates electrical energy from fuel via electrochemical reactions. A fuel cell is a. Fuel Cells Ncert.
From powerup-tech.com
The ABC of Fuel Cells PowerUP Energy Technologies Fuel Cells Ncert It is defined as an electrochemical cell that generates electrical energy from fuel via electrochemical reactions. Learn types of fuel cell, working and more here. Fuel cells use a positively charged ion (hydrogen) and an oxidizing agent (oxygen). In the presence of two electrodes, a. A fuel cell can be described as an electrochemical cell which, through an electrochemical reaction,. Fuel Cells Ncert.
From www.youtube.com
How hydrogen fuel cell works Fuel Cell Technology Working principle Fuel Cells Ncert Learn types of fuel cell, working and more here. A fuel cell can be described as an electrochemical cell which, through an electrochemical reaction, generates electrical energy from the fuel. Fuel cells use a positively charged ion (hydrogen) and an oxidizing agent (oxygen). What is a fuel cell? Electrochemistry notes class 12 covers a brief outline of topics such as. Fuel Cells Ncert.
From www.youtube.com
Trick for H2 O2 Fuel cells YouTube Fuel Cells Ncert Fuel cells use a positively charged ion (hydrogen) and an oxidizing agent (oxygen). Electrochemistry notes class 12 covers a brief outline of topics such as electrochemical cells, nernst equation, gibbs. A fuel cell can be described as an electrochemical cell which, through an electrochemical reaction, generates electrical energy from the fuel. It is defined as an electrochemical cell that generates. Fuel Cells Ncert.
From www.britannica.com
Fuel cell Definition, Types, Applications, & Facts Britannica Fuel Cells Ncert Fuel cells use a positively charged ion (hydrogen) and an oxidizing agent (oxygen). A fuel cell can be described as an electrochemical cell which, through an electrochemical reaction, generates electrical energy from the fuel. In the presence of two electrodes, a. Electrochemistry notes class 12 covers a brief outline of topics such as electrochemical cells, nernst equation, gibbs. A fuel. Fuel Cells Ncert.
From www.researchgate.net
Schematic of 61a direct methanol fuel cell (DMFC)... Download Fuel Cells Ncert Fuel cells use a positively charged ion (hydrogen) and an oxidizing agent (oxygen). A fuel cell is a tiny device, capable of generating electricity by force of a chemical reaction. Electrochemistry notes class 12 covers a brief outline of topics such as electrochemical cells, nernst equation, gibbs. A fuel cell can be described as an electrochemical cell which, through an. Fuel Cells Ncert.
From www.youtube.com
Fuel Cell Electrochemistry Chapter 2 L20 Class12 Biology(NCERT Fuel Cells Ncert A fuel cell is a tiny device, capable of generating electricity by force of a chemical reaction. A fuel cell can be described as an electrochemical cell which, through an electrochemical reaction, generates electrical energy from the fuel. What is a fuel cell? A fuel cell is a device that produces electric energy, through a chemical reaction. In the presence. Fuel Cells Ncert.