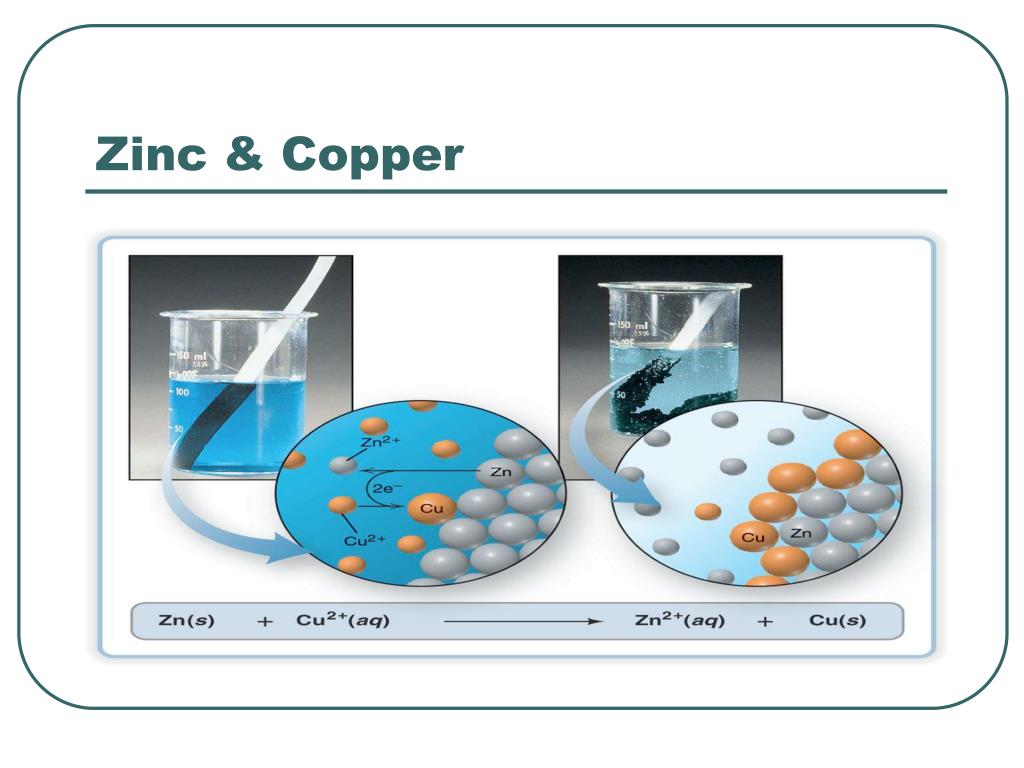
Copper And Zinc Voltage . Or, we could rely on tables of standard reduction. In the process of the. to illustrate the basic principles of a galvanic cell, let’s consider the reaction of metallic zinc with cupric ion (cu 2 +) to give copper metal and zn 2 + ion. the maximum voltage which can be produced between the poles of the cell is determined by the standard electrode. a typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the corresponding metal. The balanced chemical equation is as follows: in the zn/cu system, the valence electrons in zinc have a substantially higher potential energy than the valence electrons in. The two solutions are separated by a porous barrier that prevents them from rapidly mixing but allows ions to diffuse through. · in open circuit, the voltmeter measures the sum of these two voltages, 1.10 v, each. a simple electrochemical cell can be made from copper and zinc metals with solutions of their sulfates. zinc reduces the cu 2+ ions and copper metal plates onto the zinc.
from www.slideserve.com
to illustrate the basic principles of a galvanic cell, let’s consider the reaction of metallic zinc with cupric ion (cu 2 +) to give copper metal and zn 2 + ion. The balanced chemical equation is as follows: in the zn/cu system, the valence electrons in zinc have a substantially higher potential energy than the valence electrons in. In the process of the. a simple electrochemical cell can be made from copper and zinc metals with solutions of their sulfates. a typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the corresponding metal. zinc reduces the cu 2+ ions and copper metal plates onto the zinc. · in open circuit, the voltmeter measures the sum of these two voltages, 1.10 v, each. The two solutions are separated by a porous barrier that prevents them from rapidly mixing but allows ions to diffuse through. the maximum voltage which can be produced between the poles of the cell is determined by the standard electrode.
PPT Electrochemistry PowerPoint Presentation, free download ID5405206
Copper And Zinc Voltage in the zn/cu system, the valence electrons in zinc have a substantially higher potential energy than the valence electrons in. The balanced chemical equation is as follows: in the zn/cu system, the valence electrons in zinc have a substantially higher potential energy than the valence electrons in. to illustrate the basic principles of a galvanic cell, let’s consider the reaction of metallic zinc with cupric ion (cu 2 +) to give copper metal and zn 2 + ion. The two solutions are separated by a porous barrier that prevents them from rapidly mixing but allows ions to diffuse through. zinc reduces the cu 2+ ions and copper metal plates onto the zinc. · in open circuit, the voltmeter measures the sum of these two voltages, 1.10 v, each. a typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the corresponding metal. Or, we could rely on tables of standard reduction. the maximum voltage which can be produced between the poles of the cell is determined by the standard electrode. In the process of the. a simple electrochemical cell can be made from copper and zinc metals with solutions of their sulfates.
From colouremployer8.gitlab.io
Marvelous Copper Zinc Battery Reaction Cie Chemistry A Level Syllabus Copper And Zinc Voltage The two solutions are separated by a porous barrier that prevents them from rapidly mixing but allows ions to diffuse through. a simple electrochemical cell can be made from copper and zinc metals with solutions of their sulfates. zinc reduces the cu 2+ ions and copper metal plates onto the zinc. the maximum voltage which can be. Copper And Zinc Voltage.
From www.researchgate.net
Effect of copper concentration on cell voltage and anode potential. SO Copper And Zinc Voltage The balanced chemical equation is as follows: a typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the corresponding metal. in the zn/cu system, the valence electrons in zinc have a substantially higher potential energy than the valence electrons in. . Copper And Zinc Voltage.
From stock.adobe.com
Galvanic voltaic cell infographic diagram battery part structure Copper And Zinc Voltage Or, we could rely on tables of standard reduction. the maximum voltage which can be produced between the poles of the cell is determined by the standard electrode. to illustrate the basic principles of a galvanic cell, let’s consider the reaction of metallic zinc with cupric ion (cu 2 +) to give copper metal and zn 2 +. Copper And Zinc Voltage.
From hxenykaho.blob.core.windows.net
Copper Zinc Voltaic Cell Diagram at Paul West blog Copper And Zinc Voltage to illustrate the basic principles of a galvanic cell, let’s consider the reaction of metallic zinc with cupric ion (cu 2 +) to give copper metal and zn 2 + ion. The balanced chemical equation is as follows: In the process of the. zinc reduces the cu 2+ ions and copper metal plates onto the zinc. · in. Copper And Zinc Voltage.
From fphoto.photoshelter.com
science chemistry redox reaction copper zinc Fundamental Photographs Copper And Zinc Voltage The two solutions are separated by a porous barrier that prevents them from rapidly mixing but allows ions to diffuse through. · in open circuit, the voltmeter measures the sum of these two voltages, 1.10 v, each. a simple electrochemical cell can be made from copper and zinc metals with solutions of their sulfates. The balanced chemical equation is. Copper And Zinc Voltage.
From blog.thepipingmart.com
An Overview of Separating Copper and Zinc Alloys Copper And Zinc Voltage in the zn/cu system, the valence electrons in zinc have a substantially higher potential energy than the valence electrons in. a typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the corresponding metal. · in open circuit, the voltmeter measures the. Copper And Zinc Voltage.
From www.slideserve.com
PPT The copperzinc phase diagram Terminal and Intermediate Solid Copper And Zinc Voltage zinc reduces the cu 2+ ions and copper metal plates onto the zinc. to illustrate the basic principles of a galvanic cell, let’s consider the reaction of metallic zinc with cupric ion (cu 2 +) to give copper metal and zn 2 + ion. The balanced chemical equation is as follows: Or, we could rely on tables of. Copper And Zinc Voltage.
From quizlet.com
Label the zinc/copper galvanic cell Diagram Quizlet Copper And Zinc Voltage in the zn/cu system, the valence electrons in zinc have a substantially higher potential energy than the valence electrons in. The balanced chemical equation is as follows: the maximum voltage which can be produced between the poles of the cell is determined by the standard electrode. to illustrate the basic principles of a galvanic cell, let’s consider. Copper And Zinc Voltage.
From blog.thepipingmart.com
Classifications of Metals Copper vs. Zinc Copper And Zinc Voltage In the process of the. Or, we could rely on tables of standard reduction. The balanced chemical equation is as follows: · in open circuit, the voltmeter measures the sum of these two voltages, 1.10 v, each. a simple electrochemical cell can be made from copper and zinc metals with solutions of their sulfates. in the zn/cu system,. Copper And Zinc Voltage.
From blog.thepipingmart.com
CopperZinc Alloys A Complete Guide Copper And Zinc Voltage · in open circuit, the voltmeter measures the sum of these two voltages, 1.10 v, each. the maximum voltage which can be produced between the poles of the cell is determined by the standard electrode. The two solutions are separated by a porous barrier that prevents them from rapidly mixing but allows ions to diffuse through. Or, we could. Copper And Zinc Voltage.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID5405206 Copper And Zinc Voltage Or, we could rely on tables of standard reduction. in the zn/cu system, the valence electrons in zinc have a substantially higher potential energy than the valence electrons in. · in open circuit, the voltmeter measures the sum of these two voltages, 1.10 v, each. In the process of the. The balanced chemical equation is as follows: the. Copper And Zinc Voltage.
From pixels.com
Zinccopper Battery Photograph by Andrew Lambert Photography Copper And Zinc Voltage · in open circuit, the voltmeter measures the sum of these two voltages, 1.10 v, each. In the process of the. zinc reduces the cu 2+ ions and copper metal plates onto the zinc. the maximum voltage which can be produced between the poles of the cell is determined by the standard electrode. Or, we could rely on. Copper And Zinc Voltage.
From www.researchgate.net
Phase diagram of the copperzinc system [17]. Download Scientific Diagram Copper And Zinc Voltage · in open circuit, the voltmeter measures the sum of these two voltages, 1.10 v, each. to illustrate the basic principles of a galvanic cell, let’s consider the reaction of metallic zinc with cupric ion (cu 2 +) to give copper metal and zn 2 + ion. in the zn/cu system, the valence electrons in zinc have a. Copper And Zinc Voltage.
From www.almag.it
Characteristics of Brass and CopperZinc Alloys Almag Copper And Zinc Voltage a simple electrochemical cell can be made from copper and zinc metals with solutions of their sulfates. zinc reduces the cu 2+ ions and copper metal plates onto the zinc. the maximum voltage which can be produced between the poles of the cell is determined by the standard electrode. In the process of the. a typical. Copper And Zinc Voltage.
From blog.thepipingmart.com
Comparing the Reactivity of Copper vs Zinc Copper And Zinc Voltage In the process of the. The balanced chemical equation is as follows: a simple electrochemical cell can be made from copper and zinc metals with solutions of their sulfates. · in open circuit, the voltmeter measures the sum of these two voltages, 1.10 v, each. in the zn/cu system, the valence electrons in zinc have a substantially higher. Copper And Zinc Voltage.
From www.comsol.fr
5 W’s for 200 Years of COMSOL Blog Copper And Zinc Voltage a typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the corresponding metal. zinc reduces the cu 2+ ions and copper metal plates onto the zinc. · in open circuit, the voltmeter measures the sum of these two voltages, 1.10 v,. Copper And Zinc Voltage.
From www.slideserve.com
PPT Calculating Voltages of Electrochemical Cells PowerPoint Copper And Zinc Voltage Or, we could rely on tables of standard reduction. in the zn/cu system, the valence electrons in zinc have a substantially higher potential energy than the valence electrons in. to illustrate the basic principles of a galvanic cell, let’s consider the reaction of metallic zinc with cupric ion (cu 2 +) to give copper metal and zn 2. Copper And Zinc Voltage.
From saylordotorg.github.io
Standard Potentials Copper And Zinc Voltage the maximum voltage which can be produced between the poles of the cell is determined by the standard electrode. a simple electrochemical cell can be made from copper and zinc metals with solutions of their sulfates. in the zn/cu system, the valence electrons in zinc have a substantially higher potential energy than the valence electrons in. ·. Copper And Zinc Voltage.
From www.slideserve.com
PPT Cells and Voltage PowerPoint Presentation ID5231819 Copper And Zinc Voltage The balanced chemical equation is as follows: Or, we could rely on tables of standard reduction. zinc reduces the cu 2+ ions and copper metal plates onto the zinc. The two solutions are separated by a porous barrier that prevents them from rapidly mixing but allows ions to diffuse through. a simple electrochemical cell can be made from. Copper And Zinc Voltage.
From blog.thepipingmart.com
An Introduction to CopperZinc Alloys Copper And Zinc Voltage · in open circuit, the voltmeter measures the sum of these two voltages, 1.10 v, each. The balanced chemical equation is as follows: the maximum voltage which can be produced between the poles of the cell is determined by the standard electrode. zinc reduces the cu 2+ ions and copper metal plates onto the zinc. a typical. Copper And Zinc Voltage.
From blog.thepipingmart.com
Zinc and Copper Redox Reaction Equation Copper And Zinc Voltage to illustrate the basic principles of a galvanic cell, let’s consider the reaction of metallic zinc with cupric ion (cu 2 +) to give copper metal and zn 2 + ion. in the zn/cu system, the valence electrons in zinc have a substantially higher potential energy than the valence electrons in. the maximum voltage which can be. Copper And Zinc Voltage.
From www.chegg.com
Solved Figure below is the copper zinc phase diagram. Copper And Zinc Voltage the maximum voltage which can be produced between the poles of the cell is determined by the standard electrode. to illustrate the basic principles of a galvanic cell, let’s consider the reaction of metallic zinc with cupric ion (cu 2 +) to give copper metal and zn 2 + ion. zinc reduces the cu 2+ ions and. Copper And Zinc Voltage.
From blog.thepipingmart.com
A Breakdown of Copper vs. Zinc Reactivity Copper And Zinc Voltage a simple electrochemical cell can be made from copper and zinc metals with solutions of their sulfates. zinc reduces the cu 2+ ions and copper metal plates onto the zinc. Or, we could rely on tables of standard reduction. · in open circuit, the voltmeter measures the sum of these two voltages, 1.10 v, each. The two solutions. Copper And Zinc Voltage.
From www.youtube.com
Determination of Standard Electrode potential of Zinc and Copper YouTube Copper And Zinc Voltage a simple electrochemical cell can be made from copper and zinc metals with solutions of their sulfates. to illustrate the basic principles of a galvanic cell, let’s consider the reaction of metallic zinc with cupric ion (cu 2 +) to give copper metal and zn 2 + ion. zinc reduces the cu 2+ ions and copper metal. Copper And Zinc Voltage.
From www.alamy.com
Volta's metal arc (copper and zinc Stock Photo Alamy Copper And Zinc Voltage to illustrate the basic principles of a galvanic cell, let’s consider the reaction of metallic zinc with cupric ion (cu 2 +) to give copper metal and zn 2 + ion. the maximum voltage which can be produced between the poles of the cell is determined by the standard electrode. The balanced chemical equation is as follows: Or,. Copper And Zinc Voltage.
From brainly.in
Draw a circuit diagram showing electroplating of zinc over copper Copper And Zinc Voltage in the zn/cu system, the valence electrons in zinc have a substantially higher potential energy than the valence electrons in. · in open circuit, the voltmeter measures the sum of these two voltages, 1.10 v, each. a simple electrochemical cell can be made from copper and zinc metals with solutions of their sulfates. zinc reduces the cu. Copper And Zinc Voltage.
From blog.thepipingmart.com
Difference Between Copper and Zinc Copper And Zinc Voltage The two solutions are separated by a porous barrier that prevents them from rapidly mixing but allows ions to diffuse through. Or, we could rely on tables of standard reduction. in the zn/cu system, the valence electrons in zinc have a substantially higher potential energy than the valence electrons in. a simple electrochemical cell can be made from. Copper And Zinc Voltage.
From www.chegg.com
Solved Using the phase diagram below for brass (copperzinc Copper And Zinc Voltage to illustrate the basic principles of a galvanic cell, let’s consider the reaction of metallic zinc with cupric ion (cu 2 +) to give copper metal and zn 2 + ion. The balanced chemical equation is as follows: the maximum voltage which can be produced between the poles of the cell is determined by the standard electrode. . Copper And Zinc Voltage.
From www.nagwa.com
Question Video Calculating the Standard Cell Potential for a Copper Copper And Zinc Voltage · in open circuit, the voltmeter measures the sum of these two voltages, 1.10 v, each. Or, we could rely on tables of standard reduction. In the process of the. The balanced chemical equation is as follows: a typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution. Copper And Zinc Voltage.
From blog.thepipingmart.com
Zinc vs. Copper What's the Difference Copper And Zinc Voltage In the process of the. in the zn/cu system, the valence electrons in zinc have a substantially higher potential energy than the valence electrons in. The two solutions are separated by a porous barrier that prevents them from rapidly mixing but allows ions to diffuse through. a typical cell might consist of two pieces of metal, one zinc. Copper And Zinc Voltage.
From blog.thepipingmart.com
CopperZinc Alloys An Overview Copper And Zinc Voltage Or, we could rely on tables of standard reduction. the maximum voltage which can be produced between the poles of the cell is determined by the standard electrode. zinc reduces the cu 2+ ions and copper metal plates onto the zinc. to illustrate the basic principles of a galvanic cell, let’s consider the reaction of metallic zinc. Copper And Zinc Voltage.
From www.slideserve.com
PPT Chapter 20 PowerPoint Presentation, free download ID6976551 Copper And Zinc Voltage The two solutions are separated by a porous barrier that prevents them from rapidly mixing but allows ions to diffuse through. · in open circuit, the voltmeter measures the sum of these two voltages, 1.10 v, each. Or, we could rely on tables of standard reduction. the maximum voltage which can be produced between the poles of the cell. Copper And Zinc Voltage.
From socratic.org
Can you describe the process that releases electrons in a zinc copper Copper And Zinc Voltage In the process of the. The two solutions are separated by a porous barrier that prevents them from rapidly mixing but allows ions to diffuse through. the maximum voltage which can be produced between the poles of the cell is determined by the standard electrode. The balanced chemical equation is as follows: a simple electrochemical cell can be. Copper And Zinc Voltage.
From chemwiki.ucdavis.edu
Voltaic Cells Chemwiki Copper And Zinc Voltage Or, we could rely on tables of standard reduction. The two solutions are separated by a porous barrier that prevents them from rapidly mixing but allows ions to diffuse through. a simple electrochemical cell can be made from copper and zinc metals with solutions of their sulfates. In the process of the. the maximum voltage which can be. Copper And Zinc Voltage.
From calculator.services
Copper to Zinc Ratio Calculator Calculator Services Copper And Zinc Voltage The two solutions are separated by a porous barrier that prevents them from rapidly mixing but allows ions to diffuse through. a simple electrochemical cell can be made from copper and zinc metals with solutions of their sulfates. the maximum voltage which can be produced between the poles of the cell is determined by the standard electrode. In. Copper And Zinc Voltage.