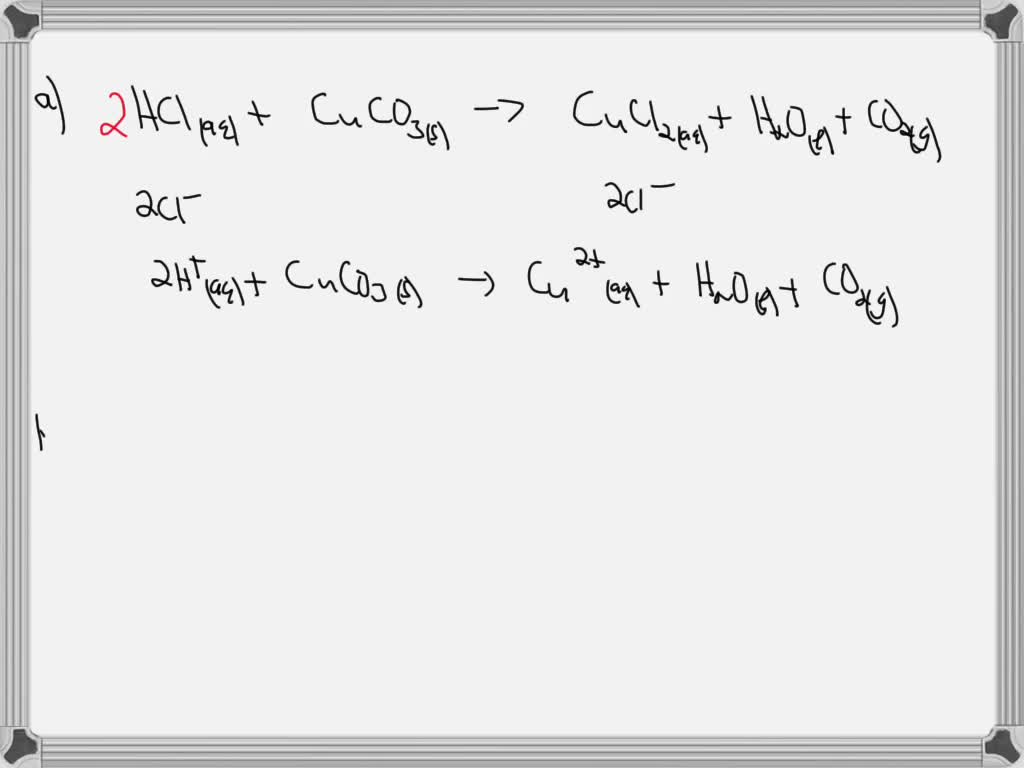Copper And Hydrochloric Acid Balanced Equation . Chemical equation (h2o = h + o) 🛠️ ${cuo + 2hcl \rightarrow cucl_2 + h_2o}$ copper oxide $(cuo)$ reacts. As ivan neretin pointed out in comments, large pieces of copper will react with hydrochloric acid over a period of several hours. For each element, we check if the number of atoms is balanced on both sides of the equation. For example, changing the reactant formula from h 2 o to h 2 o 2 would yield balance in the number of atoms, but doing so also changes the. In this specific case, the balanced chemical equation is: 1 cuo + 1 hcl = 1 cucl 2 + 1 h 2 o. In this video we'll look at the chemical equation for the reaction of hcl (hydrochloric acid). Cuo(s) + 2h+(aq) → cu2+(aq) + h2o(l) explanation: This reaction is a type of double replacement reaction called a. Use the calculator below to balance chemical equations and determine the type of reaction (instructions).
from www.numerade.com
Chemical equation (h2o = h + o) 🛠️ In this specific case, the balanced chemical equation is: This reaction is a type of double replacement reaction called a. 1 cuo + 1 hcl = 1 cucl 2 + 1 h 2 o. In this video we'll look at the chemical equation for the reaction of hcl (hydrochloric acid). ${cuo + 2hcl \rightarrow cucl_2 + h_2o}$ copper oxide $(cuo)$ reacts. For each element, we check if the number of atoms is balanced on both sides of the equation. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Cuo(s) + 2h+(aq) → cu2+(aq) + h2o(l) explanation: As ivan neretin pointed out in comments, large pieces of copper will react with hydrochloric acid over a period of several hours.
SOLVED A. Write a net ionic equation for the reaction that occurs when
Copper And Hydrochloric Acid Balanced Equation As ivan neretin pointed out in comments, large pieces of copper will react with hydrochloric acid over a period of several hours. ${cuo + 2hcl \rightarrow cucl_2 + h_2o}$ copper oxide $(cuo)$ reacts. Chemical equation (h2o = h + o) 🛠️ Cuo(s) + 2h+(aq) → cu2+(aq) + h2o(l) explanation: This reaction is a type of double replacement reaction called a. For each element, we check if the number of atoms is balanced on both sides of the equation. As ivan neretin pointed out in comments, large pieces of copper will react with hydrochloric acid over a period of several hours. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). 1 cuo + 1 hcl = 1 cucl 2 + 1 h 2 o. For example, changing the reactant formula from h 2 o to h 2 o 2 would yield balance in the number of atoms, but doing so also changes the. In this specific case, the balanced chemical equation is: In this video we'll look at the chemical equation for the reaction of hcl (hydrochloric acid).
From www.youtube.com
Type of Reaction for Cu + HCl (Copper + Hydrochloric acid) YouTube Copper And Hydrochloric Acid Balanced Equation For example, changing the reactant formula from h 2 o to h 2 o 2 would yield balance in the number of atoms, but doing so also changes the. In this specific case, the balanced chemical equation is: Chemical equation (h2o = h + o) 🛠️ Use the calculator below to balance chemical equations and determine the type of reaction. Copper And Hydrochloric Acid Balanced Equation.
From www.numerade.com
SOLVED what is the balanced equation for the reaction that occurs when Copper And Hydrochloric Acid Balanced Equation In this video we'll look at the chemical equation for the reaction of hcl (hydrochloric acid). Cuo(s) + 2h+(aq) → cu2+(aq) + h2o(l) explanation: This reaction is a type of double replacement reaction called a. 1 cuo + 1 hcl = 1 cucl 2 + 1 h 2 o. For example, changing the reactant formula from h 2 o to. Copper And Hydrochloric Acid Balanced Equation.
From www.numerade.com
SOLVED 6. Copper(II) oxide reacts with hydrochloric acid to produce Copper And Hydrochloric Acid Balanced Equation Chemical equation (h2o = h + o) 🛠️ In this specific case, the balanced chemical equation is: For example, changing the reactant formula from h 2 o to h 2 o 2 would yield balance in the number of atoms, but doing so also changes the. 1 cuo + 1 hcl = 1 cucl 2 + 1 h 2 o.. Copper And Hydrochloric Acid Balanced Equation.
From www.researchgate.net
Dissolved amounts of copper in hydrochloric and sulfuric acid solutions Copper And Hydrochloric Acid Balanced Equation In this specific case, the balanced chemical equation is: 1 cuo + 1 hcl = 1 cucl 2 + 1 h 2 o. Chemical equation (h2o = h + o) 🛠️ As ivan neretin pointed out in comments, large pieces of copper will react with hydrochloric acid over a period of several hours. This reaction is a type of double. Copper And Hydrochloric Acid Balanced Equation.
From estudiar.informacion.my.id
Cu Hcl Estudiar Copper And Hydrochloric Acid Balanced Equation For each element, we check if the number of atoms is balanced on both sides of the equation. Chemical equation (h2o = h + o) 🛠️ For example, changing the reactant formula from h 2 o to h 2 o 2 would yield balance in the number of atoms, but doing so also changes the. Cuo(s) + 2h+(aq) → cu2+(aq). Copper And Hydrochloric Acid Balanced Equation.
From www.tes.com
Ionic Equations Acids and Salts Edexcel 91 Combined Science Teaching Copper And Hydrochloric Acid Balanced Equation As ivan neretin pointed out in comments, large pieces of copper will react with hydrochloric acid over a period of several hours. In this specific case, the balanced chemical equation is: This reaction is a type of double replacement reaction called a. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). For each element,. Copper And Hydrochloric Acid Balanced Equation.
From www.slideshare.net
Chemical Reactions Copper And Hydrochloric Acid Balanced Equation Cuo(s) + 2h+(aq) → cu2+(aq) + h2o(l) explanation: In this video we'll look at the chemical equation for the reaction of hcl (hydrochloric acid). As ivan neretin pointed out in comments, large pieces of copper will react with hydrochloric acid over a period of several hours. For each element, we check if the number of atoms is balanced on both. Copper And Hydrochloric Acid Balanced Equation.
From www.chegg.com
Solved Write A Balanced Chemical Equation For Each Reacti... Copper And Hydrochloric Acid Balanced Equation As ivan neretin pointed out in comments, large pieces of copper will react with hydrochloric acid over a period of several hours. 1 cuo + 1 hcl = 1 cucl 2 + 1 h 2 o. This reaction is a type of double replacement reaction called a. For example, changing the reactant formula from h 2 o to h 2. Copper And Hydrochloric Acid Balanced Equation.
From brainly.in
Balance equation for copper ll oxide reacts with dilute hydrochloric Copper And Hydrochloric Acid Balanced Equation Use the calculator below to balance chemical equations and determine the type of reaction (instructions). 1 cuo + 1 hcl = 1 cucl 2 + 1 h 2 o. In this video we'll look at the chemical equation for the reaction of hcl (hydrochloric acid). ${cuo + 2hcl \rightarrow cucl_2 + h_2o}$ copper oxide $(cuo)$ reacts. For example, changing the. Copper And Hydrochloric Acid Balanced Equation.
From www.vrogue.co
Balance The Following Chemical Equation Caco3 Hcl Arr vrogue.co Copper And Hydrochloric Acid Balanced Equation As ivan neretin pointed out in comments, large pieces of copper will react with hydrochloric acid over a period of several hours. For example, changing the reactant formula from h 2 o to h 2 o 2 would yield balance in the number of atoms, but doing so also changes the. ${cuo + 2hcl \rightarrow cucl_2 + h_2o}$ copper oxide. Copper And Hydrochloric Acid Balanced Equation.
From www.numerade.com
SOLVED 1. Write the balanced equations for the following word Copper And Hydrochloric Acid Balanced Equation This reaction is a type of double replacement reaction called a. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). For example, changing the reactant formula from h 2 o to h 2 o 2 would yield balance in the number of atoms, but doing so also changes the. Chemical equation (h2o = h. Copper And Hydrochloric Acid Balanced Equation.
From www.numerade.com
SOLVED balanced net ionic equation for the reaction between Copper And Hydrochloric Acid Balanced Equation Use the calculator below to balance chemical equations and determine the type of reaction (instructions). In this video we'll look at the chemical equation for the reaction of hcl (hydrochloric acid). Chemical equation (h2o = h + o) 🛠️ This reaction is a type of double replacement reaction called a. In this specific case, the balanced chemical equation is: For. Copper And Hydrochloric Acid Balanced Equation.
From www.chegg.com
Write A Balanced Chemical Equation For Each Reacti... Copper And Hydrochloric Acid Balanced Equation In this specific case, the balanced chemical equation is: Use the calculator below to balance chemical equations and determine the type of reaction (instructions). As ivan neretin pointed out in comments, large pieces of copper will react with hydrochloric acid over a period of several hours. In this video we'll look at the chemical equation for the reaction of hcl. Copper And Hydrochloric Acid Balanced Equation.
From www.youtube.com
Net Ionic Equation for CuCO3 + HCl = H2O + CO2 + CuCl2 YouTube Copper And Hydrochloric Acid Balanced Equation For example, changing the reactant formula from h 2 o to h 2 o 2 would yield balance in the number of atoms, but doing so also changes the. As ivan neretin pointed out in comments, large pieces of copper will react with hydrochloric acid over a period of several hours. Cuo(s) + 2h+(aq) → cu2+(aq) + h2o(l) explanation: ${cuo. Copper And Hydrochloric Acid Balanced Equation.
From www.numerade.com
SOLVED (a) Write the balanced reaction between aqueous hydrochloric Copper And Hydrochloric Acid Balanced Equation For example, changing the reactant formula from h 2 o to h 2 o 2 would yield balance in the number of atoms, but doing so also changes the. In this video we'll look at the chemical equation for the reaction of hcl (hydrochloric acid). This reaction is a type of double replacement reaction called a. 1 cuo + 1. Copper And Hydrochloric Acid Balanced Equation.
From www.youtube.com
COPPER(II) CARBONATE & HYDROCHLORIC ACID DEMONSTRATION YouTube Copper And Hydrochloric Acid Balanced Equation 1 cuo + 1 hcl = 1 cucl 2 + 1 h 2 o. For example, changing the reactant formula from h 2 o to h 2 o 2 would yield balance in the number of atoms, but doing so also changes the. As ivan neretin pointed out in comments, large pieces of copper will react with hydrochloric acid over. Copper And Hydrochloric Acid Balanced Equation.
From www.chegg.com
Solved A. Metals with Hydrochloric Acid Copper Observation Copper And Hydrochloric Acid Balanced Equation For example, changing the reactant formula from h 2 o to h 2 o 2 would yield balance in the number of atoms, but doing so also changes the. This reaction is a type of double replacement reaction called a. Chemical equation (h2o = h + o) 🛠️ ${cuo + 2hcl \rightarrow cucl_2 + h_2o}$ copper oxide $(cuo)$ reacts. For. Copper And Hydrochloric Acid Balanced Equation.
From sciencewithmrsb.weebly.com
Acids and Bases Science with Mrs Beggs Copper And Hydrochloric Acid Balanced Equation 1 cuo + 1 hcl = 1 cucl 2 + 1 h 2 o. For example, changing the reactant formula from h 2 o to h 2 o 2 would yield balance in the number of atoms, but doing so also changes the. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). In this. Copper And Hydrochloric Acid Balanced Equation.
From www.numerade.com
SOLVEDPredict the type of reaction (if any) that occurs between each Copper And Hydrochloric Acid Balanced Equation This reaction is a type of double replacement reaction called a. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). As ivan neretin pointed out in comments, large pieces of copper will react with hydrochloric acid over a period of several hours. In this video we'll look at the chemical equation for the reaction. Copper And Hydrochloric Acid Balanced Equation.
From www.quanswer.com
When an acid reacts with metal carbonate it forms salt, carbondioxide Copper And Hydrochloric Acid Balanced Equation As ivan neretin pointed out in comments, large pieces of copper will react with hydrochloric acid over a period of several hours. In this video we'll look at the chemical equation for the reaction of hcl (hydrochloric acid). 1 cuo + 1 hcl = 1 cucl 2 + 1 h 2 o. Cuo(s) + 2h+(aq) → cu2+(aq) + h2o(l) explanation:. Copper And Hydrochloric Acid Balanced Equation.
From www.sciencephoto.com
Copper in hydrochloric acid Stock Image A500/0755 Science Photo Copper And Hydrochloric Acid Balanced Equation In this video we'll look at the chemical equation for the reaction of hcl (hydrochloric acid). As ivan neretin pointed out in comments, large pieces of copper will react with hydrochloric acid over a period of several hours. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). This reaction is a type of double. Copper And Hydrochloric Acid Balanced Equation.
From www.youtube.com
CuO+HCl=CuCl2+H2O Balanced EquationCopper oxide+Hydrochloric acid Copper And Hydrochloric Acid Balanced Equation This reaction is a type of double replacement reaction called a. As ivan neretin pointed out in comments, large pieces of copper will react with hydrochloric acid over a period of several hours. ${cuo + 2hcl \rightarrow cucl_2 + h_2o}$ copper oxide $(cuo)$ reacts. In this specific case, the balanced chemical equation is: In this video we'll look at the. Copper And Hydrochloric Acid Balanced Equation.
From www.numerade.com
SOLVED How many grams of copper (I) chloride are formed if 5.000 g of Copper And Hydrochloric Acid Balanced Equation In this video we'll look at the chemical equation for the reaction of hcl (hydrochloric acid). Cuo(s) + 2h+(aq) → cu2+(aq) + h2o(l) explanation: In this specific case, the balanced chemical equation is: This reaction is a type of double replacement reaction called a. For each element, we check if the number of atoms is balanced on both sides of. Copper And Hydrochloric Acid Balanced Equation.
From www.youtube.com
Write the balanced chemical equation of the following word equation Copper And Hydrochloric Acid Balanced Equation In this specific case, the balanced chemical equation is: Cuo(s) + 2h+(aq) → cu2+(aq) + h2o(l) explanation: ${cuo + 2hcl \rightarrow cucl_2 + h_2o}$ copper oxide $(cuo)$ reacts. For each element, we check if the number of atoms is balanced on both sides of the equation. 1 cuo + 1 hcl = 1 cucl 2 + 1 h 2 o.. Copper And Hydrochloric Acid Balanced Equation.
From www.numerade.com
SOLVED Zinc metal reacts with hydrochloric acid according to the Copper And Hydrochloric Acid Balanced Equation 1 cuo + 1 hcl = 1 cucl 2 + 1 h 2 o. ${cuo + 2hcl \rightarrow cucl_2 + h_2o}$ copper oxide $(cuo)$ reacts. This reaction is a type of double replacement reaction called a. Cuo(s) + 2h+(aq) → cu2+(aq) + h2o(l) explanation: For example, changing the reactant formula from h 2 o to h 2 o 2 would. Copper And Hydrochloric Acid Balanced Equation.
From www.youtube.com
How to Balance CuO + HCl = CuCl2 + H2O YouTube Copper And Hydrochloric Acid Balanced Equation For example, changing the reactant formula from h 2 o to h 2 o 2 would yield balance in the number of atoms, but doing so also changes the. For each element, we check if the number of atoms is balanced on both sides of the equation. This reaction is a type of double replacement reaction called a. As ivan. Copper And Hydrochloric Acid Balanced Equation.
From www.numerade.com
SOLVED Balance the equations from top to bottom 1. Acid + Hydrogen Copper And Hydrochloric Acid Balanced Equation For example, changing the reactant formula from h 2 o to h 2 o 2 would yield balance in the number of atoms, but doing so also changes the. This reaction is a type of double replacement reaction called a. Cuo(s) + 2h+(aq) → cu2+(aq) + h2o(l) explanation: In this video we'll look at the chemical equation for the reaction. Copper And Hydrochloric Acid Balanced Equation.
From www.coursehero.com
[Solved] A. copper + hydrochloric acid 1. Completion and balance the Copper And Hydrochloric Acid Balanced Equation 1 cuo + 1 hcl = 1 cucl 2 + 1 h 2 o. Chemical equation (h2o = h + o) 🛠️ In this video we'll look at the chemical equation for the reaction of hcl (hydrochloric acid). For each element, we check if the number of atoms is balanced on both sides of the equation. This reaction is a. Copper And Hydrochloric Acid Balanced Equation.
From www.advance-africa.com
Chemistry Notes Acid, Bases and Indicators Revision Notes & Tests Copper And Hydrochloric Acid Balanced Equation Chemical equation (h2o = h + o) 🛠️ Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Cuo(s) + 2h+(aq) → cu2+(aq) + h2o(l) explanation: 1 cuo + 1 hcl = 1 cucl 2 + 1 h 2 o. For each element, we check if the number of atoms is balanced on both sides. Copper And Hydrochloric Acid Balanced Equation.
From www.youtube.com
How to Write the Net Ionic Equation for CuO + HCl = CuCl2 + H2O YouTube Copper And Hydrochloric Acid Balanced Equation In this video we'll look at the chemical equation for the reaction of hcl (hydrochloric acid). 1 cuo + 1 hcl = 1 cucl 2 + 1 h 2 o. ${cuo + 2hcl \rightarrow cucl_2 + h_2o}$ copper oxide $(cuo)$ reacts. This reaction is a type of double replacement reaction called a. Chemical equation (h2o = h + o) 🛠️. Copper And Hydrochloric Acid Balanced Equation.
From www.slideserve.com
PPT DO NOW!! PowerPoint Presentation, free download ID1560113 Copper And Hydrochloric Acid Balanced Equation Use the calculator below to balance chemical equations and determine the type of reaction (instructions). For each element, we check if the number of atoms is balanced on both sides of the equation. 1 cuo + 1 hcl = 1 cucl 2 + 1 h 2 o. For example, changing the reactant formula from h 2 o to h 2. Copper And Hydrochloric Acid Balanced Equation.
From www.youtube.com
Reaction of Copper Oxide With Hydrochloric Acid YouTube Copper And Hydrochloric Acid Balanced Equation 1 cuo + 1 hcl = 1 cucl 2 + 1 h 2 o. For each element, we check if the number of atoms is balanced on both sides of the equation. In this specific case, the balanced chemical equation is: Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Cuo(s) + 2h+(aq) →. Copper And Hydrochloric Acid Balanced Equation.
From meryes.weebly.com
Balanced chemical equation with state symbols calculator meryes Copper And Hydrochloric Acid Balanced Equation Chemical equation (h2o = h + o) 🛠️ For each element, we check if the number of atoms is balanced on both sides of the equation. 1 cuo + 1 hcl = 1 cucl 2 + 1 h 2 o. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). This reaction is a type. Copper And Hydrochloric Acid Balanced Equation.
From www.chegg.com
Solved F. Other Reactions 14. Copper(II)chloride and water Copper And Hydrochloric Acid Balanced Equation 1 cuo + 1 hcl = 1 cucl 2 + 1 h 2 o. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Cuo(s) + 2h+(aq) → cu2+(aq) + h2o(l) explanation: This reaction is a type of double replacement reaction called a. Chemical equation (h2o = h + o) 🛠️ In this video we'll. Copper And Hydrochloric Acid Balanced Equation.
From www.numerade.com
SOLVED A. Write a net ionic equation for the reaction that occurs when Copper And Hydrochloric Acid Balanced Equation As ivan neretin pointed out in comments, large pieces of copper will react with hydrochloric acid over a period of several hours. For example, changing the reactant formula from h 2 o to h 2 o 2 would yield balance in the number of atoms, but doing so also changes the. Chemical equation (h2o = h + o) 🛠️ This. Copper And Hydrochloric Acid Balanced Equation.