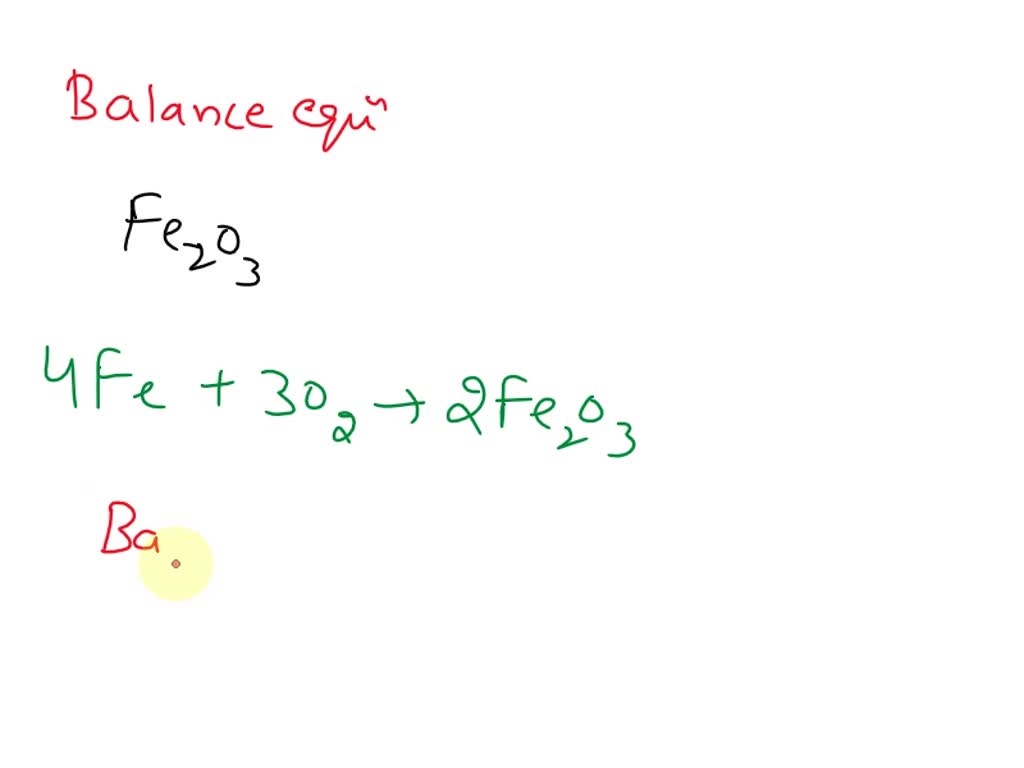Steel Wool And Oxygen Chemical Equation . steel wool consists mostly of iron. it turns out what we call rust is a chemical process that combines iron (fe) and oxygen (o) to form iron oxide. As the reaction between iron and oxygen produces iron. Write the equation for the combustion of steel wool (iron). this reaction rate demo illustrates the factor of concentration. The combustion of iron produces iron(iii) oxide, \(\ce{fe2o3}\). Write the equation for the combustion of steel wool (iron). the heat causes the iron to react with the oxygen surrounding the steel wool. The combustion of iron produces iron(iii) oxide, \(\ce{fe2o3}\). This reaction creates the spark that we see and. steel wool consists mostly of iron. since steel wool requires iron, oxygen and water to form rust, you can prevent steel wool from rusting by subtracting one part of the equation.
from www.numerade.com
steel wool consists mostly of iron. Write the equation for the combustion of steel wool (iron). As the reaction between iron and oxygen produces iron. the heat causes the iron to react with the oxygen surrounding the steel wool. This reaction creates the spark that we see and. The combustion of iron produces iron(iii) oxide, \(\ce{fe2o3}\). steel wool consists mostly of iron. it turns out what we call rust is a chemical process that combines iron (fe) and oxygen (o) to form iron oxide. this reaction rate demo illustrates the factor of concentration. The combustion of iron produces iron(iii) oxide, \(\ce{fe2o3}\).
SOLVED Explain the differences in the observations for the bottles tested with candle. Explain
Steel Wool And Oxygen Chemical Equation As the reaction between iron and oxygen produces iron. steel wool consists mostly of iron. The combustion of iron produces iron(iii) oxide, \(\ce{fe2o3}\). Write the equation for the combustion of steel wool (iron). As the reaction between iron and oxygen produces iron. The combustion of iron produces iron(iii) oxide, \(\ce{fe2o3}\). steel wool consists mostly of iron. the heat causes the iron to react with the oxygen surrounding the steel wool. Write the equation for the combustion of steel wool (iron). it turns out what we call rust is a chemical process that combines iron (fe) and oxygen (o) to form iron oxide. This reaction creates the spark that we see and. since steel wool requires iron, oxygen and water to form rust, you can prevent steel wool from rusting by subtracting one part of the equation. this reaction rate demo illustrates the factor of concentration.
From www.youtube.com
Types of Reaction Lab Reaction 3. Steel Wool + Oxygen Gas YouTube Steel Wool And Oxygen Chemical Equation steel wool consists mostly of iron. since steel wool requires iron, oxygen and water to form rust, you can prevent steel wool from rusting by subtracting one part of the equation. This reaction creates the spark that we see and. As the reaction between iron and oxygen produces iron. The combustion of iron produces iron(iii) oxide, \(\ce{fe2o3}\). . Steel Wool And Oxygen Chemical Equation.
From www.slideserve.com
PPT BALANCING CHEMICAL EQUATIONS PowerPoint Presentation, free download ID6877706 Steel Wool And Oxygen Chemical Equation The combustion of iron produces iron(iii) oxide, \(\ce{fe2o3}\). Write the equation for the combustion of steel wool (iron). This reaction creates the spark that we see and. As the reaction between iron and oxygen produces iron. it turns out what we call rust is a chemical process that combines iron (fe) and oxygen (o) to form iron oxide. The. Steel Wool And Oxygen Chemical Equation.
From www.youtube.com
Oxygen and steel wool demo YouTube Steel Wool And Oxygen Chemical Equation steel wool consists mostly of iron. As the reaction between iron and oxygen produces iron. steel wool consists mostly of iron. Write the equation for the combustion of steel wool (iron). This reaction creates the spark that we see and. The combustion of iron produces iron(iii) oxide, \(\ce{fe2o3}\). Write the equation for the combustion of steel wool (iron).. Steel Wool And Oxygen Chemical Equation.
From fyolupnrk.blob.core.windows.net
Steel Wool And Oxygen at John Becker blog Steel Wool And Oxygen Chemical Equation it turns out what we call rust is a chemical process that combines iron (fe) and oxygen (o) to form iron oxide. Write the equation for the combustion of steel wool (iron). steel wool consists mostly of iron. since steel wool requires iron, oxygen and water to form rust, you can prevent steel wool from rusting by. Steel Wool And Oxygen Chemical Equation.
From exypldlks.blob.core.windows.net
Steel Wool Chemical Equation at Robert Rhoads blog Steel Wool And Oxygen Chemical Equation The combustion of iron produces iron(iii) oxide, \(\ce{fe2o3}\). Write the equation for the combustion of steel wool (iron). The combustion of iron produces iron(iii) oxide, \(\ce{fe2o3}\). As the reaction between iron and oxygen produces iron. This reaction creates the spark that we see and. this reaction rate demo illustrates the factor of concentration. steel wool consists mostly of. Steel Wool And Oxygen Chemical Equation.
From oneclass.com
OneClass Write a balanced equation for the reaction of the steel wool with oxygen. Be sure to Steel Wool And Oxygen Chemical Equation steel wool consists mostly of iron. it turns out what we call rust is a chemical process that combines iron (fe) and oxygen (o) to form iron oxide. this reaction rate demo illustrates the factor of concentration. This reaction creates the spark that we see and. The combustion of iron produces iron(iii) oxide, \(\ce{fe2o3}\). As the reaction. Steel Wool And Oxygen Chemical Equation.
From www.numerade.com
SOLVED MuLmpLE choicequeston The main reaction occurring now that the steel wool is burning is Steel Wool And Oxygen Chemical Equation steel wool consists mostly of iron. The combustion of iron produces iron(iii) oxide, \(\ce{fe2o3}\). since steel wool requires iron, oxygen and water to form rust, you can prevent steel wool from rusting by subtracting one part of the equation. this reaction rate demo illustrates the factor of concentration. Write the equation for the combustion of steel wool. Steel Wool And Oxygen Chemical Equation.
From learningnadeaukarakas.z21.web.core.windows.net
Steel Wool Chemical Formula Steel Wool And Oxygen Chemical Equation this reaction rate demo illustrates the factor of concentration. Write the equation for the combustion of steel wool (iron). This reaction creates the spark that we see and. steel wool consists mostly of iron. steel wool consists mostly of iron. since steel wool requires iron, oxygen and water to form rust, you can prevent steel wool. Steel Wool And Oxygen Chemical Equation.
From www.slideserve.com
PPT Chemical Equations PowerPoint Presentation, free download ID6356130 Steel Wool And Oxygen Chemical Equation This reaction creates the spark that we see and. this reaction rate demo illustrates the factor of concentration. steel wool consists mostly of iron. The combustion of iron produces iron(iii) oxide, \(\ce{fe2o3}\). Write the equation for the combustion of steel wool (iron). steel wool consists mostly of iron. The combustion of iron produces iron(iii) oxide, \(\ce{fe2o3}\). . Steel Wool And Oxygen Chemical Equation.
From exybijshl.blob.core.windows.net
Steel Wool And Hcl Reaction Equation at Theodore Garcia blog Steel Wool And Oxygen Chemical Equation The combustion of iron produces iron(iii) oxide, \(\ce{fe2o3}\). As the reaction between iron and oxygen produces iron. This reaction creates the spark that we see and. since steel wool requires iron, oxygen and water to form rust, you can prevent steel wool from rusting by subtracting one part of the equation. this reaction rate demo illustrates the factor. Steel Wool And Oxygen Chemical Equation.
From www.youtube.com
Incredible steel wool combustion reaction [4K] YouTube Steel Wool And Oxygen Chemical Equation This reaction creates the spark that we see and. Write the equation for the combustion of steel wool (iron). steel wool consists mostly of iron. the heat causes the iron to react with the oxygen surrounding the steel wool. Write the equation for the combustion of steel wool (iron). it turns out what we call rust is. Steel Wool And Oxygen Chemical Equation.
From fyolupnrk.blob.core.windows.net
Steel Wool And Oxygen at John Becker blog Steel Wool And Oxygen Chemical Equation steel wool consists mostly of iron. steel wool consists mostly of iron. the heat causes the iron to react with the oxygen surrounding the steel wool. Write the equation for the combustion of steel wool (iron). Write the equation for the combustion of steel wool (iron). This reaction creates the spark that we see and. As the. Steel Wool And Oxygen Chemical Equation.
From www.chegg.com
Solved . Properties of Oxygen 1. Write word equations for Steel Wool And Oxygen Chemical Equation The combustion of iron produces iron(iii) oxide, \(\ce{fe2o3}\). steel wool consists mostly of iron. This reaction creates the spark that we see and. the heat causes the iron to react with the oxygen surrounding the steel wool. The combustion of iron produces iron(iii) oxide, \(\ce{fe2o3}\). steel wool consists mostly of iron. it turns out what we. Steel Wool And Oxygen Chemical Equation.
From www.youtube.com
Burning Steel Wool In Oxygen YouTube Steel Wool And Oxygen Chemical Equation Write the equation for the combustion of steel wool (iron). steel wool consists mostly of iron. this reaction rate demo illustrates the factor of concentration. The combustion of iron produces iron(iii) oxide, \(\ce{fe2o3}\). the heat causes the iron to react with the oxygen surrounding the steel wool. The combustion of iron produces iron(iii) oxide, \(\ce{fe2o3}\). since. Steel Wool And Oxygen Chemical Equation.
From www.researchgate.net
(PDF) STEEL WOOL RUSTING IN PURE OXYGEN ATMOSPHERE A SIMPLE EXPERIMENT Steel Wool And Oxygen Chemical Equation it turns out what we call rust is a chemical process that combines iron (fe) and oxygen (o) to form iron oxide. steel wool consists mostly of iron. The combustion of iron produces iron(iii) oxide, \(\ce{fe2o3}\). this reaction rate demo illustrates the factor of concentration. the heat causes the iron to react with the oxygen surrounding. Steel Wool And Oxygen Chemical Equation.
From www.slideserve.com
PPT Reacting metals with oxygen PowerPoint Presentation, free download ID6645971 Steel Wool And Oxygen Chemical Equation Write the equation for the combustion of steel wool (iron). steel wool consists mostly of iron. since steel wool requires iron, oxygen and water to form rust, you can prevent steel wool from rusting by subtracting one part of the equation. the heat causes the iron to react with the oxygen surrounding the steel wool. steel. Steel Wool And Oxygen Chemical Equation.
From www.sciencephoto.com
Steel Wool Burning in Oxygen Stock Image C036/3398 Science Photo Library Steel Wool And Oxygen Chemical Equation As the reaction between iron and oxygen produces iron. Write the equation for the combustion of steel wool (iron). steel wool consists mostly of iron. since steel wool requires iron, oxygen and water to form rust, you can prevent steel wool from rusting by subtracting one part of the equation. The combustion of iron produces iron(iii) oxide, \(\ce{fe2o3}\).. Steel Wool And Oxygen Chemical Equation.
From learning.physics.iastate.edu
Steel Wool and Liquid Oxygen Physics Demonstrations Steel Wool And Oxygen Chemical Equation This reaction creates the spark that we see and. Write the equation for the combustion of steel wool (iron). The combustion of iron produces iron(iii) oxide, \(\ce{fe2o3}\). steel wool consists mostly of iron. Write the equation for the combustion of steel wool (iron). The combustion of iron produces iron(iii) oxide, \(\ce{fe2o3}\). since steel wool requires iron, oxygen and. Steel Wool And Oxygen Chemical Equation.
From hxegskbdu.blob.core.windows.net
Burning Steel Wool Equation at Josephine Luken blog Steel Wool And Oxygen Chemical Equation Write the equation for the combustion of steel wool (iron). since steel wool requires iron, oxygen and water to form rust, you can prevent steel wool from rusting by subtracting one part of the equation. the heat causes the iron to react with the oxygen surrounding the steel wool. The combustion of iron produces iron(iii) oxide, \(\ce{fe2o3}\). . Steel Wool And Oxygen Chemical Equation.
From fphoto.photoshelter.com
science chemistry exothermic reaction steel wool oxygen Fundamental Photographs The Art of Steel Wool And Oxygen Chemical Equation steel wool consists mostly of iron. The combustion of iron produces iron(iii) oxide, \(\ce{fe2o3}\). As the reaction between iron and oxygen produces iron. This reaction creates the spark that we see and. since steel wool requires iron, oxygen and water to form rust, you can prevent steel wool from rusting by subtracting one part of the equation. . Steel Wool And Oxygen Chemical Equation.
From www.numerade.com
SOLVED Write the balanced chemical reaction for the rusting of iron? the acidic vinegar Steel Wool And Oxygen Chemical Equation The combustion of iron produces iron(iii) oxide, \(\ce{fe2o3}\). the heat causes the iron to react with the oxygen surrounding the steel wool. this reaction rate demo illustrates the factor of concentration. it turns out what we call rust is a chemical process that combines iron (fe) and oxygen (o) to form iron oxide. Write the equation for. Steel Wool And Oxygen Chemical Equation.
From www.upstartmag.co.nz
Steel Wool Chemical Reaction Experiment for Kids — Upstart Magazine Steel Wool And Oxygen Chemical Equation Write the equation for the combustion of steel wool (iron). This reaction creates the spark that we see and. The combustion of iron produces iron(iii) oxide, \(\ce{fe2o3}\). steel wool consists mostly of iron. As the reaction between iron and oxygen produces iron. steel wool consists mostly of iron. since steel wool requires iron, oxygen and water to. Steel Wool And Oxygen Chemical Equation.
From fphoto.photoshelter.com
science chemistry exothermic reaction steel wool oxygen Fundamental Photographs The Art of Steel Wool And Oxygen Chemical Equation it turns out what we call rust is a chemical process that combines iron (fe) and oxygen (o) to form iron oxide. This reaction creates the spark that we see and. As the reaction between iron and oxygen produces iron. since steel wool requires iron, oxygen and water to form rust, you can prevent steel wool from rusting. Steel Wool And Oxygen Chemical Equation.
From www.upstartmag.co.nz
Steel Wool Chemical Reaction Experiment for Kids — Upstart Magazine Steel Wool And Oxygen Chemical Equation Write the equation for the combustion of steel wool (iron). the heat causes the iron to react with the oxygen surrounding the steel wool. steel wool consists mostly of iron. This reaction creates the spark that we see and. it turns out what we call rust is a chemical process that combines iron (fe) and oxygen (o). Steel Wool And Oxygen Chemical Equation.
From exybijshl.blob.core.windows.net
Steel Wool And Hcl Reaction Equation at Theodore Garcia blog Steel Wool And Oxygen Chemical Equation steel wool consists mostly of iron. This reaction creates the spark that we see and. steel wool consists mostly of iron. since steel wool requires iron, oxygen and water to form rust, you can prevent steel wool from rusting by subtracting one part of the equation. Write the equation for the combustion of steel wool (iron). . Steel Wool And Oxygen Chemical Equation.
From fphoto.photoshelter.com
science chemistry exothermic reaction steel wool oxygen Fundamental Photographs The Art of Steel Wool And Oxygen Chemical Equation The combustion of iron produces iron(iii) oxide, \(\ce{fe2o3}\). The combustion of iron produces iron(iii) oxide, \(\ce{fe2o3}\). This reaction creates the spark that we see and. this reaction rate demo illustrates the factor of concentration. Write the equation for the combustion of steel wool (iron). the heat causes the iron to react with the oxygen surrounding the steel wool.. Steel Wool And Oxygen Chemical Equation.
From www.numerade.com
SOLVED Explain the differences in the observations for the bottles tested with candle. Explain Steel Wool And Oxygen Chemical Equation The combustion of iron produces iron(iii) oxide, \(\ce{fe2o3}\). This reaction creates the spark that we see and. steel wool consists mostly of iron. this reaction rate demo illustrates the factor of concentration. Write the equation for the combustion of steel wool (iron). As the reaction between iron and oxygen produces iron. Write the equation for the combustion of. Steel Wool And Oxygen Chemical Equation.
From www.slideserve.com
PPT BALANCING CHEMICAL EQUATIONS PowerPoint Presentation, free download ID6877706 Steel Wool And Oxygen Chemical Equation The combustion of iron produces iron(iii) oxide, \(\ce{fe2o3}\). Write the equation for the combustion of steel wool (iron). The combustion of iron produces iron(iii) oxide, \(\ce{fe2o3}\). the heat causes the iron to react with the oxygen surrounding the steel wool. This reaction creates the spark that we see and. steel wool consists mostly of iron. it turns. Steel Wool And Oxygen Chemical Equation.
From upstartmag.squarespace.com
Steel Wool Chemical Reaction Experiment for Kids — Upstart Magazine Steel Wool And Oxygen Chemical Equation steel wool consists mostly of iron. Write the equation for the combustion of steel wool (iron). this reaction rate demo illustrates the factor of concentration. the heat causes the iron to react with the oxygen surrounding the steel wool. The combustion of iron produces iron(iii) oxide, \(\ce{fe2o3}\). This reaction creates the spark that we see and. The. Steel Wool And Oxygen Chemical Equation.
From www.numerade.com
SOLVED In this reaction, iron (steel wool) is combining with oxygen to form iron (III) oxide Steel Wool And Oxygen Chemical Equation it turns out what we call rust is a chemical process that combines iron (fe) and oxygen (o) to form iron oxide. The combustion of iron produces iron(iii) oxide, \(\ce{fe2o3}\). As the reaction between iron and oxygen produces iron. since steel wool requires iron, oxygen and water to form rust, you can prevent steel wool from rusting by. Steel Wool And Oxygen Chemical Equation.
From www.slideserve.com
PPT BALANCING CHEMICAL EQUATIONS PowerPoint Presentation, free download ID6877706 Steel Wool And Oxygen Chemical Equation since steel wool requires iron, oxygen and water to form rust, you can prevent steel wool from rusting by subtracting one part of the equation. As the reaction between iron and oxygen produces iron. Write the equation for the combustion of steel wool (iron). This reaction creates the spark that we see and. it turns out what we. Steel Wool And Oxygen Chemical Equation.
From answerzonecelluloid.z21.web.core.windows.net
Steel Wool Chemical Formula Steel Wool And Oxygen Chemical Equation Write the equation for the combustion of steel wool (iron). steel wool consists mostly of iron. since steel wool requires iron, oxygen and water to form rust, you can prevent steel wool from rusting by subtracting one part of the equation. Write the equation for the combustion of steel wool (iron). steel wool consists mostly of iron.. Steel Wool And Oxygen Chemical Equation.
From teachingscience.us
Combustion Reaction Burning Steel Wool Teaching Science with Lynda R. Williams Steel Wool And Oxygen Chemical Equation steel wool consists mostly of iron. Write the equation for the combustion of steel wool (iron). This reaction creates the spark that we see and. the heat causes the iron to react with the oxygen surrounding the steel wool. Write the equation for the combustion of steel wool (iron). since steel wool requires iron, oxygen and water. Steel Wool And Oxygen Chemical Equation.
From teachingscience.us
Combustion Reaction Burning Steel Wool Teaching Science with Lynda R. Williams Steel Wool And Oxygen Chemical Equation As the reaction between iron and oxygen produces iron. steel wool consists mostly of iron. Write the equation for the combustion of steel wool (iron). The combustion of iron produces iron(iii) oxide, \(\ce{fe2o3}\). The combustion of iron produces iron(iii) oxide, \(\ce{fe2o3}\). since steel wool requires iron, oxygen and water to form rust, you can prevent steel wool from. Steel Wool And Oxygen Chemical Equation.
From www.numerade.com
SOLVED Steel wool reacts with oxygen in the synthesis reaction in activity 1. Answer the Steel Wool And Oxygen Chemical Equation it turns out what we call rust is a chemical process that combines iron (fe) and oxygen (o) to form iron oxide. The combustion of iron produces iron(iii) oxide, \(\ce{fe2o3}\). steel wool consists mostly of iron. The combustion of iron produces iron(iii) oxide, \(\ce{fe2o3}\). This reaction creates the spark that we see and. Write the equation for the. Steel Wool And Oxygen Chemical Equation.