Titration Of Koh And H2So4 . in the ch3cooh/naoh titration, that would be when one mole of naoh has been added to one mole of ch3cooh. This results in the formation of two moles of water (h 2 o) and one mole of sodium sulfate (na 2 so 4). our titration calculator will help you never have to ask how do i calculate titrations?. in this process, 2 moles (the molecular weight of a substance expressed in grams) of sodium hydroxide (naoh) combine with one mole of sulfuric acid (h 2 so 4). chemistry,general chemistry,science tutorial,chemistry tutorial,titration,acid,base,stoichiometry,moles,liters,concentration,molarity,volume,acid. in a titration of sulfuric acid against sodium hydroxide, \(32.20 \: A student titrated a 25.0 cm3 sample of sulfuric acid, h2so4, with a 0.102 mol/dm3 solution of. a titration’s end point was determined using litmus as an indicator, which is red in acidic solutions and blue in.
from www.numerade.com
in this process, 2 moles (the molecular weight of a substance expressed in grams) of sodium hydroxide (naoh) combine with one mole of sulfuric acid (h 2 so 4). in a titration of sulfuric acid against sodium hydroxide, \(32.20 \: A student titrated a 25.0 cm3 sample of sulfuric acid, h2so4, with a 0.102 mol/dm3 solution of. our titration calculator will help you never have to ask how do i calculate titrations?. chemistry,general chemistry,science tutorial,chemistry tutorial,titration,acid,base,stoichiometry,moles,liters,concentration,molarity,volume,acid. This results in the formation of two moles of water (h 2 o) and one mole of sodium sulfate (na 2 so 4). a titration’s end point was determined using litmus as an indicator, which is red in acidic solutions and blue in. in the ch3cooh/naoh titration, that would be when one mole of naoh has been added to one mole of ch3cooh.
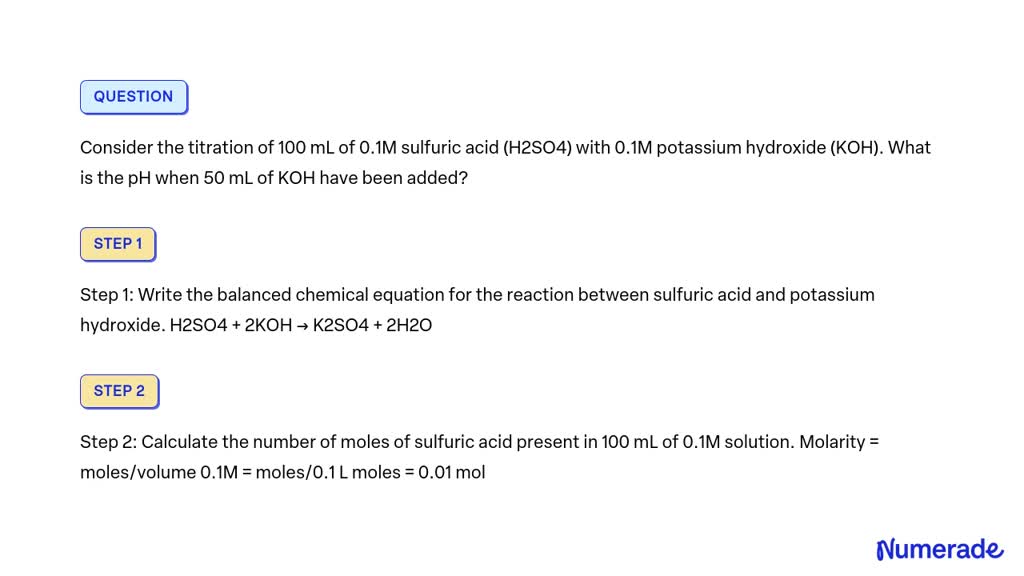
SOLVED Consider the titration of 100 mL of 0.1M sulfuric acid (H2SO4) with 0.1M potassium
Titration Of Koh And H2So4 in this process, 2 moles (the molecular weight of a substance expressed in grams) of sodium hydroxide (naoh) combine with one mole of sulfuric acid (h 2 so 4). This results in the formation of two moles of water (h 2 o) and one mole of sodium sulfate (na 2 so 4). our titration calculator will help you never have to ask how do i calculate titrations?. in this process, 2 moles (the molecular weight of a substance expressed in grams) of sodium hydroxide (naoh) combine with one mole of sulfuric acid (h 2 so 4). chemistry,general chemistry,science tutorial,chemistry tutorial,titration,acid,base,stoichiometry,moles,liters,concentration,molarity,volume,acid. in a titration of sulfuric acid against sodium hydroxide, \(32.20 \: in the ch3cooh/naoh titration, that would be when one mole of naoh has been added to one mole of ch3cooh. A student titrated a 25.0 cm3 sample of sulfuric acid, h2so4, with a 0.102 mol/dm3 solution of. a titration’s end point was determined using litmus as an indicator, which is red in acidic solutions and blue in.
From www.youtube.com
Titration Reaction H2SO4 and LiOH YouTube Titration Of Koh And H2So4 in this process, 2 moles (the molecular weight of a substance expressed in grams) of sodium hydroxide (naoh) combine with one mole of sulfuric acid (h 2 so 4). A student titrated a 25.0 cm3 sample of sulfuric acid, h2so4, with a 0.102 mol/dm3 solution of. in the ch3cooh/naoh titration, that would be when one mole of naoh. Titration Of Koh And H2So4.
From giotpdccx.blob.core.windows.net
H2So4 Titration With Naoh Calculation at Selene Larsen blog Titration Of Koh And H2So4 our titration calculator will help you never have to ask how do i calculate titrations?. in the ch3cooh/naoh titration, that would be when one mole of naoh has been added to one mole of ch3cooh. chemistry,general chemistry,science tutorial,chemistry tutorial,titration,acid,base,stoichiometry,moles,liters,concentration,molarity,volume,acid. This results in the formation of two moles of water (h 2 o) and one mole of sodium. Titration Of Koh And H2So4.
From giomzfpyz.blob.core.windows.net
Titration Of Naoh By H2So4 at Judith Phillips blog Titration Of Koh And H2So4 chemistry,general chemistry,science tutorial,chemistry tutorial,titration,acid,base,stoichiometry,moles,liters,concentration,molarity,volume,acid. in this process, 2 moles (the molecular weight of a substance expressed in grams) of sodium hydroxide (naoh) combine with one mole of sulfuric acid (h 2 so 4). a titration’s end point was determined using litmus as an indicator, which is red in acidic solutions and blue in. in a titration. Titration Of Koh And H2So4.
From www.chegg.com
Solved A student carried out a titration using H2SO4 and Titration Of Koh And H2So4 a titration’s end point was determined using litmus as an indicator, which is red in acidic solutions and blue in. A student titrated a 25.0 cm3 sample of sulfuric acid, h2so4, with a 0.102 mol/dm3 solution of. This results in the formation of two moles of water (h 2 o) and one mole of sodium sulfate (na 2 so. Titration Of Koh And H2So4.
From mavink.com
H2so4 Titration Curve Titration Of Koh And H2So4 This results in the formation of two moles of water (h 2 o) and one mole of sodium sulfate (na 2 so 4). our titration calculator will help you never have to ask how do i calculate titrations?. A student titrated a 25.0 cm3 sample of sulfuric acid, h2so4, with a 0.102 mol/dm3 solution of. in this process,. Titration Of Koh And H2So4.
From slideplayer.com
Acid Base Titrations Lesson ppt download Titration Of Koh And H2So4 in a titration of sulfuric acid against sodium hydroxide, \(32.20 \: in this process, 2 moles (the molecular weight of a substance expressed in grams) of sodium hydroxide (naoh) combine with one mole of sulfuric acid (h 2 so 4). A student titrated a 25.0 cm3 sample of sulfuric acid, h2so4, with a 0.102 mol/dm3 solution of. This. Titration Of Koh And H2So4.
From www.numerade.com
SOLVED Write the balanced equation for the neutralization reaction between H2SO4 and KOH in Titration Of Koh And H2So4 A student titrated a 25.0 cm3 sample of sulfuric acid, h2so4, with a 0.102 mol/dm3 solution of. in the ch3cooh/naoh titration, that would be when one mole of naoh has been added to one mole of ch3cooh. chemistry,general chemistry,science tutorial,chemistry tutorial,titration,acid,base,stoichiometry,moles,liters,concentration,molarity,volume,acid. our titration calculator will help you never have to ask how do i calculate titrations?. . Titration Of Koh And H2So4.
From www.chegg.com
Solved When an acid is titrated with a KOH solution, the Titration Of Koh And H2So4 in the ch3cooh/naoh titration, that would be when one mole of naoh has been added to one mole of ch3cooh. a titration’s end point was determined using litmus as an indicator, which is red in acidic solutions and blue in. This results in the formation of two moles of water (h 2 o) and one mole of sodium. Titration Of Koh And H2So4.
From www.studypool.com
SOLUTION Solution for a student carried out a titration using h2so4 and koh the balanced Titration Of Koh And H2So4 in this process, 2 moles (the molecular weight of a substance expressed in grams) of sodium hydroxide (naoh) combine with one mole of sulfuric acid (h 2 so 4). our titration calculator will help you never have to ask how do i calculate titrations?. in the ch3cooh/naoh titration, that would be when one mole of naoh has. Titration Of Koh And H2So4.
From www.numerade.com
SOLVED Acidbase titration 03 Hilal was asked to obtain the concentration of H2SO4 in a given Titration Of Koh And H2So4 a titration’s end point was determined using litmus as an indicator, which is red in acidic solutions and blue in. in the ch3cooh/naoh titration, that would be when one mole of naoh has been added to one mole of ch3cooh. in this process, 2 moles (the molecular weight of a substance expressed in grams) of sodium hydroxide. Titration Of Koh And H2So4.
From mavink.com
Titration Labeled Titration Of Koh And H2So4 our titration calculator will help you never have to ask how do i calculate titrations?. A student titrated a 25.0 cm3 sample of sulfuric acid, h2so4, with a 0.102 mol/dm3 solution of. chemistry,general chemistry,science tutorial,chemistry tutorial,titration,acid,base,stoichiometry,moles,liters,concentration,molarity,volume,acid. in the ch3cooh/naoh titration, that would be when one mole of naoh has been added to one mole of ch3cooh. This. Titration Of Koh And H2So4.
From mungfali.com
The Titration Of 25 0 Ml Of An Unknown Concentration Of H2so4 Solution 699 Titration Of Koh And H2So4 in the ch3cooh/naoh titration, that would be when one mole of naoh has been added to one mole of ch3cooh. This results in the formation of two moles of water (h 2 o) and one mole of sodium sulfate (na 2 so 4). in this process, 2 moles (the molecular weight of a substance expressed in grams) of. Titration Of Koh And H2So4.
From www.youtube.com
Balance KOH + H2SO4 = K2SO4 + H2O (Potassium Hydroxide and Sulfuric Acid) YouTube Titration Of Koh And H2So4 our titration calculator will help you never have to ask how do i calculate titrations?. in the ch3cooh/naoh titration, that would be when one mole of naoh has been added to one mole of ch3cooh. in a titration of sulfuric acid against sodium hydroxide, \(32.20 \: chemistry,general chemistry,science tutorial,chemistry tutorial,titration,acid,base,stoichiometry,moles,liters,concentration,molarity,volume,acid. This results in the formation of. Titration Of Koh And H2So4.
From www.numerade.com
SOLVED Student carried out a titration experiment to titrate 30 mL of sulfuric acid H2SO4 of Titration Of Koh And H2So4 a titration’s end point was determined using litmus as an indicator, which is red in acidic solutions and blue in. in a titration of sulfuric acid against sodium hydroxide, \(32.20 \: A student titrated a 25.0 cm3 sample of sulfuric acid, h2so4, with a 0.102 mol/dm3 solution of. in the ch3cooh/naoh titration, that would be when one. Titration Of Koh And H2So4.
From www.numerade.com
SOLVED Consider the titration of 100 mL of 0.1M sulfuric acid (H2SO4) with 0.1M potassium Titration Of Koh And H2So4 in the ch3cooh/naoh titration, that would be when one mole of naoh has been added to one mole of ch3cooh. A student titrated a 25.0 cm3 sample of sulfuric acid, h2so4, with a 0.102 mol/dm3 solution of. our titration calculator will help you never have to ask how do i calculate titrations?. a titration’s end point was. Titration Of Koh And H2So4.
From www.numerade.com
SOLVED A 4A2i.5mL sample of a KOH solution of unknown concentration requires 16 mL of 0.150 M Titration Of Koh And H2So4 in the ch3cooh/naoh titration, that would be when one mole of naoh has been added to one mole of ch3cooh. A student titrated a 25.0 cm3 sample of sulfuric acid, h2so4, with a 0.102 mol/dm3 solution of. in a titration of sulfuric acid against sodium hydroxide, \(32.20 \: This results in the formation of two moles of water. Titration Of Koh And H2So4.
From oneclass.com
OneClass Write the balanced neutralization reaction between H2SO4 and KOH in aqueous solution Titration Of Koh And H2So4 A student titrated a 25.0 cm3 sample of sulfuric acid, h2so4, with a 0.102 mol/dm3 solution of. This results in the formation of two moles of water (h 2 o) and one mole of sodium sulfate (na 2 so 4). in the ch3cooh/naoh titration, that would be when one mole of naoh has been added to one mole of. Titration Of Koh And H2So4.
From www.youtube.com
How to Write the Net Ionic Equation for KOH + H2SO4 = K2SO4 + H2O YouTube Titration Of Koh And H2So4 in this process, 2 moles (the molecular weight of a substance expressed in grams) of sodium hydroxide (naoh) combine with one mole of sulfuric acid (h 2 so 4). in the ch3cooh/naoh titration, that would be when one mole of naoh has been added to one mole of ch3cooh. in a titration of sulfuric acid against sodium. Titration Of Koh And H2So4.
From www.slideshare.net
4 to post 1 Titration Of Koh And H2So4 a titration’s end point was determined using litmus as an indicator, which is red in acidic solutions and blue in. in the ch3cooh/naoh titration, that would be when one mole of naoh has been added to one mole of ch3cooh. This results in the formation of two moles of water (h 2 o) and one mole of sodium. Titration Of Koh And H2So4.
From www.numerade.com
SOLVED During a titration, 10.00 mL of sulphuric acid, H2SO4 is titrated with 0.625 mol/L Titration Of Koh And H2So4 This results in the formation of two moles of water (h 2 o) and one mole of sodium sulfate (na 2 so 4). a titration’s end point was determined using litmus as an indicator, which is red in acidic solutions and blue in. in the ch3cooh/naoh titration, that would be when one mole of naoh has been added. Titration Of Koh And H2So4.
From www.youtube.com
Type of Reaction for KOH + H2SO4 = K2SO4 + H2O YouTube Titration Of Koh And H2So4 chemistry,general chemistry,science tutorial,chemistry tutorial,titration,acid,base,stoichiometry,moles,liters,concentration,molarity,volume,acid. A student titrated a 25.0 cm3 sample of sulfuric acid, h2so4, with a 0.102 mol/dm3 solution of. in this process, 2 moles (the molecular weight of a substance expressed in grams) of sodium hydroxide (naoh) combine with one mole of sulfuric acid (h 2 so 4). in a titration of sulfuric acid against. Titration Of Koh And H2So4.
From www.numerade.com
SOLVED 'The reaction between potassium hydroxide and sulfuric acid is given below. If you have Titration Of Koh And H2So4 chemistry,general chemistry,science tutorial,chemistry tutorial,titration,acid,base,stoichiometry,moles,liters,concentration,molarity,volume,acid. our titration calculator will help you never have to ask how do i calculate titrations?. in a titration of sulfuric acid against sodium hydroxide, \(32.20 \: A student titrated a 25.0 cm3 sample of sulfuric acid, h2so4, with a 0.102 mol/dm3 solution of. in the ch3cooh/naoh titration, that would be when one. Titration Of Koh And H2So4.
From www.studypool.com
SOLUTION Estimation of koh with standardized h2so4 titration calculation Studypool Titration Of Koh And H2So4 This results in the formation of two moles of water (h 2 o) and one mole of sodium sulfate (na 2 so 4). our titration calculator will help you never have to ask how do i calculate titrations?. a titration’s end point was determined using litmus as an indicator, which is red in acidic solutions and blue in.. Titration Of Koh And H2So4.
From www.numerade.com
A student carried out a titration using H2SO4 and KOH. The balanced equation for the reaction is Titration Of Koh And H2So4 a titration’s end point was determined using litmus as an indicator, which is red in acidic solutions and blue in. chemistry,general chemistry,science tutorial,chemistry tutorial,titration,acid,base,stoichiometry,moles,liters,concentration,molarity,volume,acid. in the ch3cooh/naoh titration, that would be when one mole of naoh has been added to one mole of ch3cooh. our titration calculator will help you never have to ask how do. Titration Of Koh And H2So4.
From www.numerade.com
SOLVED The titration of a 20.0mL sample of an H2SO4 solution of unknown concentration requires Titration Of Koh And H2So4 A student titrated a 25.0 cm3 sample of sulfuric acid, h2so4, with a 0.102 mol/dm3 solution of. This results in the formation of two moles of water (h 2 o) and one mole of sodium sulfate (na 2 so 4). our titration calculator will help you never have to ask how do i calculate titrations?. in the ch3cooh/naoh. Titration Of Koh And H2So4.
From www.numerade.com
SOLVED Using the following data of the results of a titration with potassium hydroxide (KOH Titration Of Koh And H2So4 A student titrated a 25.0 cm3 sample of sulfuric acid, h2so4, with a 0.102 mol/dm3 solution of. This results in the formation of two moles of water (h 2 o) and one mole of sodium sulfate (na 2 so 4). in this process, 2 moles (the molecular weight of a substance expressed in grams) of sodium hydroxide (naoh) combine. Titration Of Koh And H2So4.
From www.youtube.com
KOH+H2SO4=K2SO4+H2O Balance the chemical equation mydocumentary838. koh+h2so4=k2so4+h2o YouTube Titration Of Koh And H2So4 This results in the formation of two moles of water (h 2 o) and one mole of sodium sulfate (na 2 so 4). a titration’s end point was determined using litmus as an indicator, which is red in acidic solutions and blue in. A student titrated a 25.0 cm3 sample of sulfuric acid, h2so4, with a 0.102 mol/dm3 solution. Titration Of Koh And H2So4.
From www.researchgate.net
Titration of 1 mL diluted bath (H2SO4/H3PO4) with 0.5 M NaOH in water... Download Scientific Titration Of Koh And H2So4 chemistry,general chemistry,science tutorial,chemistry tutorial,titration,acid,base,stoichiometry,moles,liters,concentration,molarity,volume,acid. in a titration of sulfuric acid against sodium hydroxide, \(32.20 \: a titration’s end point was determined using litmus as an indicator, which is red in acidic solutions and blue in. in the ch3cooh/naoh titration, that would be when one mole of naoh has been added to one mole of ch3cooh. This. Titration Of Koh And H2So4.
From www.numerade.com
SOLVED Describe what happens at the molecular level when the equivalence point is reached Titration Of Koh And H2So4 This results in the formation of two moles of water (h 2 o) and one mole of sodium sulfate (na 2 so 4). a titration’s end point was determined using litmus as an indicator, which is red in acidic solutions and blue in. our titration calculator will help you never have to ask how do i calculate titrations?.. Titration Of Koh And H2So4.
From www.numerade.com
SOLVED Part A Avolume of 70.0 mL of aqueous potassium hydroxide (KOH) was titrated against Titration Of Koh And H2So4 a titration’s end point was determined using litmus as an indicator, which is red in acidic solutions and blue in. in a titration of sulfuric acid against sodium hydroxide, \(32.20 \: This results in the formation of two moles of water (h 2 o) and one mole of sodium sulfate (na 2 so 4). in this process,. Titration Of Koh And H2So4.
From www.numerade.com
Determine the pH during the titration of 30 mL of 3.0M KOH with 1.5M H2SO4 after the addition of Titration Of Koh And H2So4 a titration’s end point was determined using litmus as an indicator, which is red in acidic solutions and blue in. A student titrated a 25.0 cm3 sample of sulfuric acid, h2so4, with a 0.102 mol/dm3 solution of. in the ch3cooh/naoh titration, that would be when one mole of naoh has been added to one mole of ch3cooh. . Titration Of Koh And H2So4.
From www.youtube.com
Balance KOH + H2SO4 = K2SO4 + H2O (Potassium Hydroxide and Sulfuric Acid) YouTube Titration Of Koh And H2So4 A student titrated a 25.0 cm3 sample of sulfuric acid, h2so4, with a 0.102 mol/dm3 solution of. in a titration of sulfuric acid against sodium hydroxide, \(32.20 \: in this process, 2 moles (the molecular weight of a substance expressed in grams) of sodium hydroxide (naoh) combine with one mole of sulfuric acid (h 2 so 4). . Titration Of Koh And H2So4.
From www.youtube.com
KOH AND H2SO4 TITRATION YouTube Titration Of Koh And H2So4 chemistry,general chemistry,science tutorial,chemistry tutorial,titration,acid,base,stoichiometry,moles,liters,concentration,molarity,volume,acid. a titration’s end point was determined using litmus as an indicator, which is red in acidic solutions and blue in. in the ch3cooh/naoh titration, that would be when one mole of naoh has been added to one mole of ch3cooh. in this process, 2 moles (the molecular weight of a substance expressed. Titration Of Koh And H2So4.
From mavink.com
H2so4 Titration Curve Titration Of Koh And H2So4 A student titrated a 25.0 cm3 sample of sulfuric acid, h2so4, with a 0.102 mol/dm3 solution of. chemistry,general chemistry,science tutorial,chemistry tutorial,titration,acid,base,stoichiometry,moles,liters,concentration,molarity,volume,acid. a titration’s end point was determined using litmus as an indicator, which is red in acidic solutions and blue in. in this process, 2 moles (the molecular weight of a substance expressed in grams) of sodium. Titration Of Koh And H2So4.
From www.numerade.com
SOLVED give the spectator ions for the reactions that occur when aqueous solutions of H2SO4 and Titration Of Koh And H2So4 a titration’s end point was determined using litmus as an indicator, which is red in acidic solutions and blue in. This results in the formation of two moles of water (h 2 o) and one mole of sodium sulfate (na 2 so 4). our titration calculator will help you never have to ask how do i calculate titrations?.. Titration Of Koh And H2So4.