What Is The Process Of Gas . learn about the four main changes of state: drifting smoke particles indicate the movement of the surrounding gas. Find out how to use heating. when an ideal gas is compressed adiabatically \((q = 0)\), work is done on it and its temperature increases; Gas is one of the four fundamental states of matter.the others are solid, liquid, and. learn about the relationship between ideal gases and thermodynamics, and how to calculate the internal energy, heat, and work of. learn about the six phase changes between solids, liquids, and gases, and the eight phase changes if you include plasma. learn how liquid water changes to gas (steam) when heated and how gas changes to liquid (condensation) when. Melting, freezing, evaporating and condensing.
from www.mdpi.com
learn about the four main changes of state: when an ideal gas is compressed adiabatically \((q = 0)\), work is done on it and its temperature increases; learn about the relationship between ideal gases and thermodynamics, and how to calculate the internal energy, heat, and work of. Gas is one of the four fundamental states of matter.the others are solid, liquid, and. drifting smoke particles indicate the movement of the surrounding gas. Find out how to use heating. learn how liquid water changes to gas (steam) when heated and how gas changes to liquid (condensation) when. Melting, freezing, evaporating and condensing. learn about the six phase changes between solids, liquids, and gases, and the eight phase changes if you include plasma.
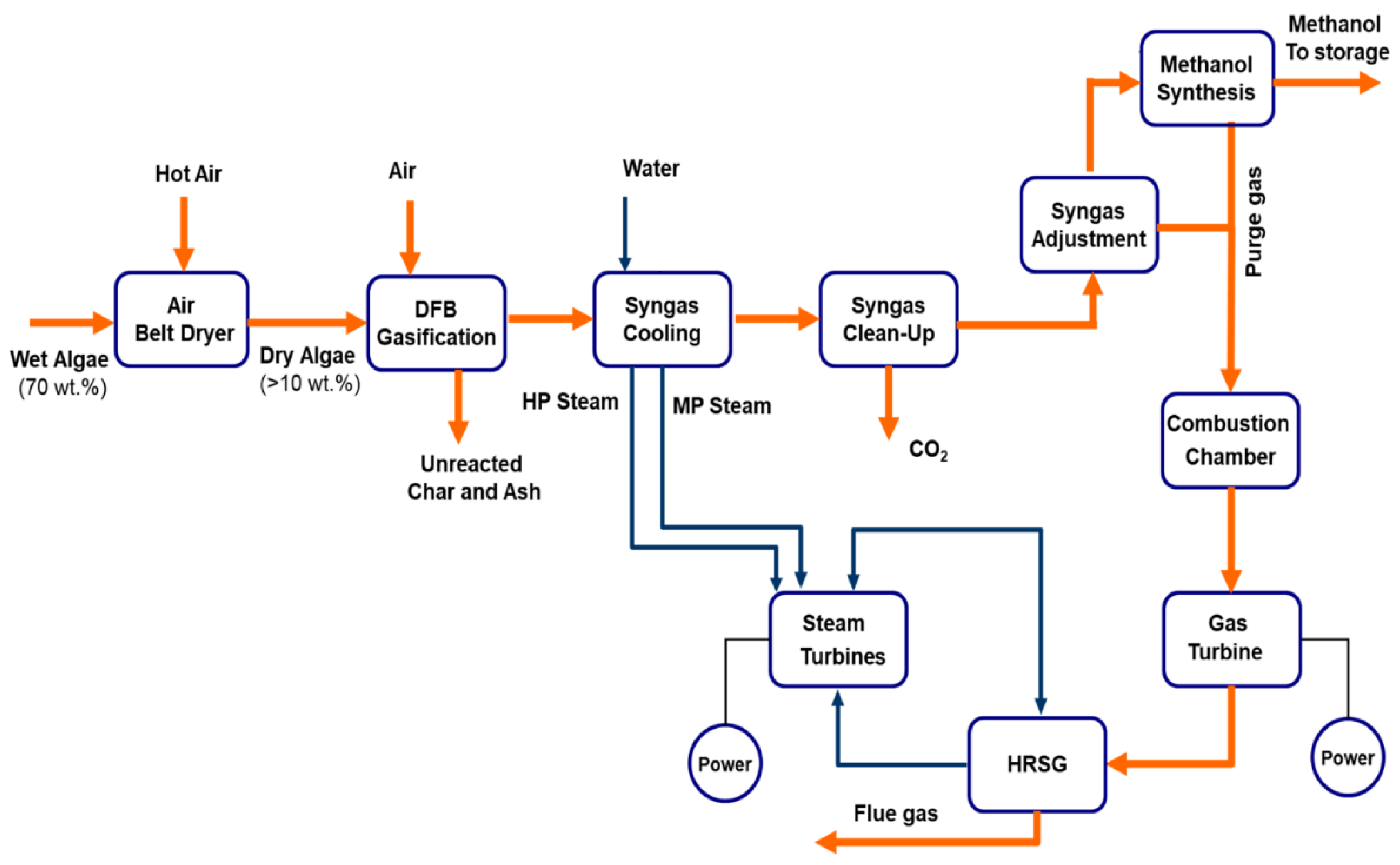
Energies Free FullText Development of a Biomass Gasification
What Is The Process Of Gas learn how liquid water changes to gas (steam) when heated and how gas changes to liquid (condensation) when. Gas is one of the four fundamental states of matter.the others are solid, liquid, and. learn about the four main changes of state: learn about the six phase changes between solids, liquids, and gases, and the eight phase changes if you include plasma. learn how liquid water changes to gas (steam) when heated and how gas changes to liquid (condensation) when. when an ideal gas is compressed adiabatically \((q = 0)\), work is done on it and its temperature increases; learn about the relationship between ideal gases and thermodynamics, and how to calculate the internal energy, heat, and work of. Find out how to use heating. Melting, freezing, evaporating and condensing. drifting smoke particles indicate the movement of the surrounding gas.
From www.youtube.com
Gas to liquids Process YouTube What Is The Process Of Gas when an ideal gas is compressed adiabatically \((q = 0)\), work is done on it and its temperature increases; learn about the relationship between ideal gases and thermodynamics, and how to calculate the internal energy, heat, and work of. learn about the six phase changes between solids, liquids, and gases, and the eight phase changes if you. What Is The Process Of Gas.
From www.dreamstime.com
Illustration for Changes of State between Solid, Liquid and Gas Stock What Is The Process Of Gas learn about the four main changes of state: learn about the relationship between ideal gases and thermodynamics, and how to calculate the internal energy, heat, and work of. when an ideal gas is compressed adiabatically \((q = 0)\), work is done on it and its temperature increases; drifting smoke particles indicate the movement of the surrounding. What Is The Process Of Gas.
From exoczxabo.blob.core.windows.net
Producer Gas Definition at James Greene blog What Is The Process Of Gas learn about the six phase changes between solids, liquids, and gases, and the eight phase changes if you include plasma. when an ideal gas is compressed adiabatically \((q = 0)\), work is done on it and its temperature increases; learn about the relationship between ideal gases and thermodynamics, and how to calculate the internal energy, heat, and. What Is The Process Of Gas.
From chemicalengineeringworld.com
Gas Absorption Process Chemical Engineering World What Is The Process Of Gas when an ideal gas is compressed adiabatically \((q = 0)\), work is done on it and its temperature increases; learn about the six phase changes between solids, liquids, and gases, and the eight phase changes if you include plasma. learn about the relationship between ideal gases and thermodynamics, and how to calculate the internal energy, heat, and. What Is The Process Of Gas.
From sciencenotes.org
Examples of Gases What Is a Gas? What Is The Process Of Gas Melting, freezing, evaporating and condensing. learn how liquid water changes to gas (steam) when heated and how gas changes to liquid (condensation) when. learn about the relationship between ideal gases and thermodynamics, and how to calculate the internal energy, heat, and work of. drifting smoke particles indicate the movement of the surrounding gas. learn about the. What Is The Process Of Gas.
From www.slideserve.com
PPT Plant Processes Gas Exchange and Photosynthesis PowerPoint What Is The Process Of Gas learn about the relationship between ideal gases and thermodynamics, and how to calculate the internal energy, heat, and work of. Find out how to use heating. learn about the four main changes of state: drifting smoke particles indicate the movement of the surrounding gas. Gas is one of the four fundamental states of matter.the others are solid,. What Is The Process Of Gas.
From www.dreamstime.com
4 Processes in Gasification. Education Infographic. Vector Design What Is The Process Of Gas Find out how to use heating. learn about the relationship between ideal gases and thermodynamics, and how to calculate the internal energy, heat, and work of. learn about the six phase changes between solids, liquids, and gases, and the eight phase changes if you include plasma. learn about the four main changes of state: when an. What Is The Process Of Gas.
From www.researchgate.net
Process flow diagram for natural gas sweetening by absorption using What Is The Process Of Gas drifting smoke particles indicate the movement of the surrounding gas. learn about the four main changes of state: learn about the relationship between ideal gases and thermodynamics, and how to calculate the internal energy, heat, and work of. Find out how to use heating. Gas is one of the four fundamental states of matter.the others are solid,. What Is The Process Of Gas.
From www.researchgate.net
Schematic of the methanol synthesis process. The hydrogen/syngas What Is The Process Of Gas when an ideal gas is compressed adiabatically \((q = 0)\), work is done on it and its temperature increases; drifting smoke particles indicate the movement of the surrounding gas. Gas is one of the four fundamental states of matter.the others are solid, liquid, and. learn about the six phase changes between solids, liquids, and gases, and the. What Is The Process Of Gas.
From www.slideserve.com
PPT Plant Processes Gas Exchange and Photosynthesis PowerPoint What Is The Process Of Gas when an ideal gas is compressed adiabatically \((q = 0)\), work is done on it and its temperature increases; learn about the four main changes of state: Melting, freezing, evaporating and condensing. drifting smoke particles indicate the movement of the surrounding gas. learn about the six phase changes between solids, liquids, and gases, and the eight. What Is The Process Of Gas.
From www.mdpi.com
Energies Free FullText Development of a Biomass Gasification What Is The Process Of Gas when an ideal gas is compressed adiabatically \((q = 0)\), work is done on it and its temperature increases; Melting, freezing, evaporating and condensing. Find out how to use heating. learn how liquid water changes to gas (steam) when heated and how gas changes to liquid (condensation) when. learn about the six phase changes between solids, liquids,. What Is The Process Of Gas.
From www.mdpi.com
Gases Free FullText Carbon Capture from CO2Rich Natural Gas via What Is The Process Of Gas learn how liquid water changes to gas (steam) when heated and how gas changes to liquid (condensation) when. when an ideal gas is compressed adiabatically \((q = 0)\), work is done on it and its temperature increases; learn about the four main changes of state: learn about the six phase changes between solids, liquids, and gases,. What Is The Process Of Gas.
From www.dreamstime.com
Evaporation Vector Illustration. Labeled Liquid To Gas State Process What Is The Process Of Gas drifting smoke particles indicate the movement of the surrounding gas. Gas is one of the four fundamental states of matter.the others are solid, liquid, and. when an ideal gas is compressed adiabatically \((q = 0)\), work is done on it and its temperature increases; learn how liquid water changes to gas (steam) when heated and how gas. What Is The Process Of Gas.
From mungfali.com
Gas Plant Process Flow Diagram What Is The Process Of Gas learn about the four main changes of state: Melting, freezing, evaporating and condensing. learn about the six phase changes between solids, liquids, and gases, and the eight phase changes if you include plasma. drifting smoke particles indicate the movement of the surrounding gas. learn about the relationship between ideal gases and thermodynamics, and how to calculate. What Is The Process Of Gas.
From www.tec-science.com
Van der Waals equation (gas law for real gases) tecscience What Is The Process Of Gas learn about the relationship between ideal gases and thermodynamics, and how to calculate the internal energy, heat, and work of. drifting smoke particles indicate the movement of the surrounding gas. Find out how to use heating. learn how liquid water changes to gas (steam) when heated and how gas changes to liquid (condensation) when. learn about. What Is The Process Of Gas.
From www.slideserve.com
PPT Plant Processes Gas Exchange and Photosynthesis PowerPoint What Is The Process Of Gas drifting smoke particles indicate the movement of the surrounding gas. when an ideal gas is compressed adiabatically \((q = 0)\), work is done on it and its temperature increases; Find out how to use heating. Melting, freezing, evaporating and condensing. Gas is one of the four fundamental states of matter.the others are solid, liquid, and. learn about. What Is The Process Of Gas.
From climatechange.chicago.gov
Global Greenhouse Gas Emissions Data Greenhouse Gas (GHG) Emissions What Is The Process Of Gas Gas is one of the four fundamental states of matter.the others are solid, liquid, and. drifting smoke particles indicate the movement of the surrounding gas. learn about the six phase changes between solids, liquids, and gases, and the eight phase changes if you include plasma. Find out how to use heating. when an ideal gas is compressed. What Is The Process Of Gas.
From www.aerzen.com
Process gas technology What Is The Process Of Gas learn about the four main changes of state: Gas is one of the four fundamental states of matter.the others are solid, liquid, and. learn about the relationship between ideal gases and thermodynamics, and how to calculate the internal energy, heat, and work of. drifting smoke particles indicate the movement of the surrounding gas. Melting, freezing, evaporating and. What Is The Process Of Gas.
From www.e-education.psu.edu
Acid Gas Removal FSC 432 Petroleum Refining What Is The Process Of Gas Melting, freezing, evaporating and condensing. learn how liquid water changes to gas (steam) when heated and how gas changes to liquid (condensation) when. when an ideal gas is compressed adiabatically \((q = 0)\), work is done on it and its temperature increases; learn about the six phase changes between solids, liquids, and gases, and the eight phase. What Is The Process Of Gas.
From www.uky.edu
Chemicals from Coal Gasification, Kentucky Geological Survey What Is The Process Of Gas learn about the relationship between ideal gases and thermodynamics, and how to calculate the internal energy, heat, and work of. Find out how to use heating. Gas is one of the four fundamental states of matter.the others are solid, liquid, and. drifting smoke particles indicate the movement of the surrounding gas. when an ideal gas is compressed. What Is The Process Of Gas.
From www.sciencekids.co.nz
Volcanic Gases Diagram Nature & Environment Pictures, Images What Is The Process Of Gas learn about the six phase changes between solids, liquids, and gases, and the eight phase changes if you include plasma. Find out how to use heating. Gas is one of the four fundamental states of matter.the others are solid, liquid, and. learn about the relationship between ideal gases and thermodynamics, and how to calculate the internal energy, heat,. What Is The Process Of Gas.
From mavink.com
Gas Plant Process Flow Diagram What Is The Process Of Gas learn about the six phase changes between solids, liquids, and gases, and the eight phase changes if you include plasma. Find out how to use heating. learn how liquid water changes to gas (steam) when heated and how gas changes to liquid (condensation) when. learn about the relationship between ideal gases and thermodynamics, and how to calculate. What Is The Process Of Gas.
From mavink.com
Oil And Gas Process Flow Diagram What Is The Process Of Gas learn how liquid water changes to gas (steam) when heated and how gas changes to liquid (condensation) when. when an ideal gas is compressed adiabatically \((q = 0)\), work is done on it and its temperature increases; learn about the four main changes of state: Gas is one of the four fundamental states of matter.the others are. What Is The Process Of Gas.
From www.youtube.com
Gas Processing Plant Process Flow Diagram and Explanation YouTube What Is The Process Of Gas learn about the six phase changes between solids, liquids, and gases, and the eight phase changes if you include plasma. Gas is one of the four fundamental states of matter.the others are solid, liquid, and. learn about the four main changes of state: drifting smoke particles indicate the movement of the surrounding gas. learn about the. What Is The Process Of Gas.
From ar.inspiredpencil.com
Natural Gas Diagram For Kids What Is The Process Of Gas learn about the relationship between ideal gases and thermodynamics, and how to calculate the internal energy, heat, and work of. when an ideal gas is compressed adiabatically \((q = 0)\), work is done on it and its temperature increases; drifting smoke particles indicate the movement of the surrounding gas. learn about the four main changes of. What Is The Process Of Gas.
From vectormine.com
Human gas exchange process with oxygen cycle explanation outline What Is The Process Of Gas learn how liquid water changes to gas (steam) when heated and how gas changes to liquid (condensation) when. learn about the four main changes of state: drifting smoke particles indicate the movement of the surrounding gas. when an ideal gas is compressed adiabatically \((q = 0)\), work is done on it and its temperature increases; Find. What Is The Process Of Gas.
From www.snexplores.org
Explainer What are the different states of matter? What Is The Process Of Gas Find out how to use heating. learn how liquid water changes to gas (steam) when heated and how gas changes to liquid (condensation) when. learn about the relationship between ideal gases and thermodynamics, and how to calculate the internal energy, heat, and work of. Gas is one of the four fundamental states of matter.the others are solid, liquid,. What Is The Process Of Gas.
From tie-tech.com
Theory of Gas Injection Processes Tietech What Is The Process Of Gas drifting smoke particles indicate the movement of the surrounding gas. learn about the four main changes of state: learn how liquid water changes to gas (steam) when heated and how gas changes to liquid (condensation) when. learn about the six phase changes between solids, liquids, and gases, and the eight phase changes if you include plasma.. What Is The Process Of Gas.
From eoncoat.com
How to eliminate corrosion in petrochemical facilities? What Is The Process Of Gas Find out how to use heating. Gas is one of the four fundamental states of matter.the others are solid, liquid, and. learn about the six phase changes between solids, liquids, and gases, and the eight phase changes if you include plasma. learn about the relationship between ideal gases and thermodynamics, and how to calculate the internal energy, heat,. What Is The Process Of Gas.
From stock.adobe.com
Human gas exchange process with oxygen cycle explanation outline What Is The Process Of Gas learn about the four main changes of state: learn how liquid water changes to gas (steam) when heated and how gas changes to liquid (condensation) when. drifting smoke particles indicate the movement of the surrounding gas. Find out how to use heating. when an ideal gas is compressed adiabatically \((q = 0)\), work is done on. What Is The Process Of Gas.
From www.teachoo.com
[Chemistry Class 10] (a) Identify gasses evolved at anode and cathode What Is The Process Of Gas learn how liquid water changes to gas (steam) when heated and how gas changes to liquid (condensation) when. drifting smoke particles indicate the movement of the surrounding gas. learn about the four main changes of state: Melting, freezing, evaporating and condensing. Find out how to use heating. learn about the six phase changes between solids, liquids,. What Is The Process Of Gas.
From www.dreamstime.com
Gas Diffusion Phenomenon of Oxygen and Hydrogen Stock Vector What Is The Process Of Gas drifting smoke particles indicate the movement of the surrounding gas. learn how liquid water changes to gas (steam) when heated and how gas changes to liquid (condensation) when. Find out how to use heating. learn about the six phase changes between solids, liquids, and gases, and the eight phase changes if you include plasma. Gas is one. What Is The Process Of Gas.
From www.youtube.com
molecular theory of gases Physical Processes MCAT Khan What Is The Process Of Gas learn how liquid water changes to gas (steam) when heated and how gas changes to liquid (condensation) when. learn about the relationship between ideal gases and thermodynamics, and how to calculate the internal energy, heat, and work of. Gas is one of the four fundamental states of matter.the others are solid, liquid, and. Melting, freezing, evaporating and condensing.. What Is The Process Of Gas.
From processflowsheets.blogspot.com
Process flow sheets Natural gas processing with flow chart What Is The Process Of Gas drifting smoke particles indicate the movement of the surrounding gas. learn how liquid water changes to gas (steam) when heated and how gas changes to liquid (condensation) when. learn about the relationship between ideal gases and thermodynamics, and how to calculate the internal energy, heat, and work of. learn about the six phase changes between solids,. What Is The Process Of Gas.
From www.thenile.co.nz
Process Gas Chromatographs by Tony Waters, Hardcover, 9781119633044 What Is The Process Of Gas learn about the six phase changes between solids, liquids, and gases, and the eight phase changes if you include plasma. Gas is one of the four fundamental states of matter.the others are solid, liquid, and. Melting, freezing, evaporating and condensing. drifting smoke particles indicate the movement of the surrounding gas. learn about the four main changes of. What Is The Process Of Gas.