Average Heat Capacity Of Calorimeter . Additionally, it can find the. In both cases, the amount of heat absorbed or released by the calorimeter is equal in magnitude and opposite in sign to the amount of heat produced or consumed by the reaction. The temperature increase is measured and, along with the known heat capacity of the calorimeter, is used to calculate the energy produced by the. (use 4.184 j g¯ 1 °c¯ 1 as the specific heat of water.) solution: A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat capacity) is. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature. If $\pu{1.25 g}$ of glucose are burnt in a calorimeter containing $\pu{0.95 kg}$ of. Heat always flows from high temperature to low temperature. 1) heat given up by warm. The calorimetry calculator can help you solve complex calorimetry problems. The amount of heat absorbed or released (q) by the object depends on its mass. The combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. Calculate the heat capacity of the calorimeter in j/°c. It can analyze the heat exchange between up to 3 objects.
from www.numerade.com
Additionally, it can find the. The combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. In both cases, the amount of heat absorbed or released by the calorimeter is equal in magnitude and opposite in sign to the amount of heat produced or consumed by the reaction. The amount of heat absorbed or released (q) by the object depends on its mass. 1) heat given up by warm. If $\pu{1.25 g}$ of glucose are burnt in a calorimeter containing $\pu{0.95 kg}$ of. The calorimetry calculator can help you solve complex calorimetry problems. Heat always flows from high temperature to low temperature. Calculate the heat capacity of the calorimeter in j/°c. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature.
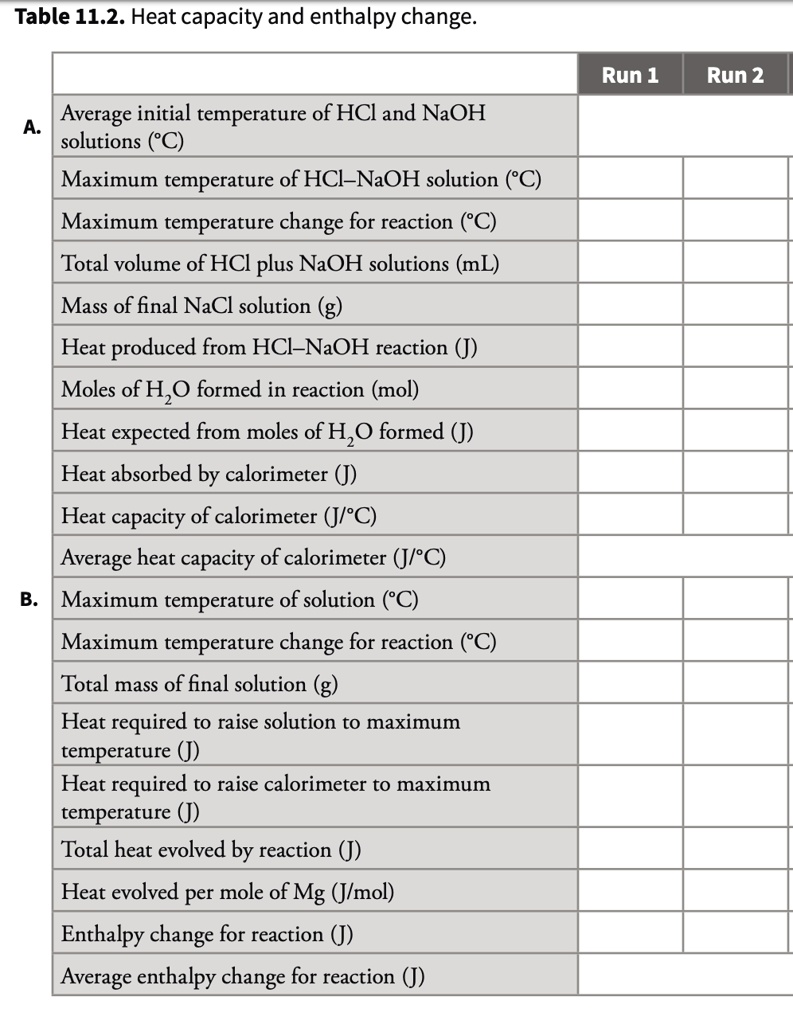
Table 11.2 Heat Capacity and Enthalpy Change Run 1 Run 2 Average
Average Heat Capacity Of Calorimeter In both cases, the amount of heat absorbed or released by the calorimeter is equal in magnitude and opposite in sign to the amount of heat produced or consumed by the reaction. Additionally, it can find the. The temperature increase is measured and, along with the known heat capacity of the calorimeter, is used to calculate the energy produced by the. (use 4.184 j g¯ 1 °c¯ 1 as the specific heat of water.) solution: Heat always flows from high temperature to low temperature. If $\pu{1.25 g}$ of glucose are burnt in a calorimeter containing $\pu{0.95 kg}$ of. It can analyze the heat exchange between up to 3 objects. 1) heat given up by warm. In both cases, the amount of heat absorbed or released by the calorimeter is equal in magnitude and opposite in sign to the amount of heat produced or consumed by the reaction. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature. The calorimetry calculator can help you solve complex calorimetry problems. The amount of heat absorbed or released (q) by the object depends on its mass. A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat capacity) is. Calculate the heat capacity of the calorimeter in j/°c. The combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat.
From www.chegg.com
Solved Calculations I. Heat Capacity of the Calorimeter The Average Heat Capacity Of Calorimeter If $\pu{1.25 g}$ of glucose are burnt in a calorimeter containing $\pu{0.95 kg}$ of. In both cases, the amount of heat absorbed or released by the calorimeter is equal in magnitude and opposite in sign to the amount of heat produced or consumed by the reaction. (use 4.184 j g¯ 1 °c¯ 1 as the specific heat of water.) solution:. Average Heat Capacity Of Calorimeter.
From www.chegg.com
Solved Part A Heat Capacity of the Calorimeter (1 Average Heat Capacity Of Calorimeter Calculate the heat capacity of the calorimeter in j/°c. (use 4.184 j g¯ 1 °c¯ 1 as the specific heat of water.) solution: The combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. 1) heat given up by warm. The calorimetry calculator can help you solve complex calorimetry problems. The temperature increase is measured and, along with. Average Heat Capacity Of Calorimeter.
From www.chegg.com
Part A Heat Capacity of the Calorimeter (1 Average Heat Capacity Of Calorimeter The combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. Additionally, it can find the. (use 4.184 j g¯ 1 °c¯ 1 as the specific heat of water.) solution: The temperature increase is measured and, along with the known heat capacity of the calorimeter, is used to calculate the energy produced by the. The amount of heat. Average Heat Capacity Of Calorimeter.
From www.animalia-life.club
Calorimeter Diagram Average Heat Capacity Of Calorimeter The calorimetry calculator can help you solve complex calorimetry problems. It can analyze the heat exchange between up to 3 objects. Additionally, it can find the. The combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. The amount of heat absorbed or released (q) by the object depends on its mass. 1) heat given up by warm.. Average Heat Capacity Of Calorimeter.
From saylordotorg.github.io
Calorimetry Average Heat Capacity Of Calorimeter The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature. A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat capacity) is. 1) heat given up by warm.. Average Heat Capacity Of Calorimeter.
From www.numerade.com
SOLVED Table 1 Determining the heat capacity of the calorimeter. Run Average Heat Capacity Of Calorimeter The amount of heat absorbed or released (q) by the object depends on its mass. (use 4.184 j g¯ 1 °c¯ 1 as the specific heat of water.) solution: It can analyze the heat exchange between up to 3 objects. If $\pu{1.25 g}$ of glucose are burnt in a calorimeter containing $\pu{0.95 kg}$ of. A container that prevents heat transfer. Average Heat Capacity Of Calorimeter.
From www.scienceabc.com
Molar Heat Capacity Definition, Formula, Equation, Calculation Average Heat Capacity Of Calorimeter In both cases, the amount of heat absorbed or released by the calorimeter is equal in magnitude and opposite in sign to the amount of heat produced or consumed by the reaction. Calculate the heat capacity of the calorimeter in j/°c. The temperature increase is measured and, along with the known heat capacity of the calorimeter, is used to calculate. Average Heat Capacity Of Calorimeter.
From www.chegg.com
Solved Heat capacity of calorimeter (C cal), using the Average Heat Capacity Of Calorimeter In both cases, the amount of heat absorbed or released by the calorimeter is equal in magnitude and opposite in sign to the amount of heat produced or consumed by the reaction. The combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. The calorimetry calculator can help you solve complex calorimetry problems. Additionally, it can find the.. Average Heat Capacity Of Calorimeter.
From 2012books.lardbucket.org
Calorimetry Average Heat Capacity Of Calorimeter The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature. The temperature increase is measured and, along with the known heat capacity of the calorimeter, is used to calculate the energy produced by the. If $\pu{1.25 g}$ of glucose are burnt in a calorimeter containing $\pu{0.95. Average Heat Capacity Of Calorimeter.
From webapi.bu.edu
⚡ The cold equations analysis. The Cold Equation By Tom Godwin Analysis Average Heat Capacity Of Calorimeter The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature. Additionally, it can find the. Calculate the heat capacity of the calorimeter in j/°c. The calorimetry calculator can help you solve complex calorimetry problems. In both cases, the amount of heat absorbed or released by the. Average Heat Capacity Of Calorimeter.
From www.youtube.com
CH5 Q5 Calculating the Heat Capacity of a Calorimeter YouTube Average Heat Capacity Of Calorimeter Calculate the heat capacity of the calorimeter in j/°c. (use 4.184 j g¯ 1 °c¯ 1 as the specific heat of water.) solution: The amount of heat absorbed or released (q) by the object depends on its mass. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences. Average Heat Capacity Of Calorimeter.
From www.chegg.com
Solved 1. Heat Lost by Water in J to calorimeter. 2. Average Heat Capacity Of Calorimeter Additionally, it can find the. The temperature increase is measured and, along with the known heat capacity of the calorimeter, is used to calculate the energy produced by the. If $\pu{1.25 g}$ of glucose are burnt in a calorimeter containing $\pu{0.95 kg}$ of. (use 4.184 j g¯ 1 °c¯ 1 as the specific heat of water.) solution: A container that. Average Heat Capacity Of Calorimeter.
From www.chegg.com
Solved connect Calorimetry TRY. HEAT Average Heat Capacity Of Calorimeter Calculate the heat capacity of the calorimeter in j/°c. In both cases, the amount of heat absorbed or released by the calorimeter is equal in magnitude and opposite in sign to the amount of heat produced or consumed by the reaction. The amount of heat absorbed or released (q) by the object depends on its mass. The combustion of 1. Average Heat Capacity Of Calorimeter.
From www.bartleby.com
Answered Table 1 Calorimeter Heat Capacity… bartleby Average Heat Capacity Of Calorimeter The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature. A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat capacity) is. Heat always flows from high temperature. Average Heat Capacity Of Calorimeter.
From www.youtube.com
CHEMISTRY 101 Specific heat capacity and calculating heat YouTube Average Heat Capacity Of Calorimeter (use 4.184 j g¯ 1 °c¯ 1 as the specific heat of water.) solution: If $\pu{1.25 g}$ of glucose are burnt in a calorimeter containing $\pu{0.95 kg}$ of. A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat capacity) is. Calculate the. Average Heat Capacity Of Calorimeter.
From www.chegg.com
Solved ACTIVITY 1 A Heat Capacity of the Calorimeter 1. Average Heat Capacity Of Calorimeter It can analyze the heat exchange between up to 3 objects. The amount of heat absorbed or released (q) by the object depends on its mass. The calorimetry calculator can help you solve complex calorimetry problems. (use 4.184 j g¯ 1 °c¯ 1 as the specific heat of water.) solution: Heat always flows from high temperature to low temperature. Additionally,. Average Heat Capacity Of Calorimeter.
From www.chegg.com
Solved Calculate the heat capacity, as described on page 5 Average Heat Capacity Of Calorimeter The combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat capacity) is. The temperature increase is measured and, along with the known heat capacity of the calorimeter, is used. Average Heat Capacity Of Calorimeter.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec Average Heat Capacity Of Calorimeter Heat always flows from high temperature to low temperature. The calorimetry calculator can help you solve complex calorimetry problems. If $\pu{1.25 g}$ of glucose are burnt in a calorimeter containing $\pu{0.95 kg}$ of. (use 4.184 j g¯ 1 °c¯ 1 as the specific heat of water.) solution: In both cases, the amount of heat absorbed or released by the calorimeter. Average Heat Capacity Of Calorimeter.
From www.chegg.com
Solved Part A Heat capacity calculations Run 1 Run 2 Average Heat Capacity Of Calorimeter Additionally, it can find the. Heat always flows from high temperature to low temperature. (use 4.184 j g¯ 1 °c¯ 1 as the specific heat of water.) solution: The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature. In both cases, the amount of heat absorbed. Average Heat Capacity Of Calorimeter.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Average Heat Capacity Of Calorimeter The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature. The combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. If $\pu{1.25 g}$ of glucose are burnt in a calorimeter containing $\pu{0.95 kg}$ of. The amount of heat absorbed or released (q) by. Average Heat Capacity Of Calorimeter.
From www.chegg.com
Solved Part A Heat capacity calculations Run 1 Run 2 Average Heat Capacity Of Calorimeter It can analyze the heat exchange between up to 3 objects. Additionally, it can find the. The amount of heat absorbed or released (q) by the object depends on its mass. Calculate the heat capacity of the calorimeter in j/°c. Heat always flows from high temperature to low temperature. (use 4.184 j g¯ 1 °c¯ 1 as the specific heat. Average Heat Capacity Of Calorimeter.
From www.chegg.com
Solved Calculate Heat capacity of the calorimeter (J/ ºC) , Average Heat Capacity Of Calorimeter The combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. In both cases, the amount of heat absorbed or released by the calorimeter is equal in magnitude and opposite in sign to the amount of heat produced or consumed by the reaction. 1) heat given up by warm. (use 4.184 j g¯ 1 °c¯ 1 as the. Average Heat Capacity Of Calorimeter.
From www.chegg.com
Solved In the laboratory a "coffee cup" calorimeter, or Average Heat Capacity Of Calorimeter Calculate the heat capacity of the calorimeter in j/°c. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature. (use 4.184 j g¯ 1 °c¯ 1 as the specific heat of water.) solution: The amount of heat absorbed or released (q) by the object depends on. Average Heat Capacity Of Calorimeter.
From www.youtube.com
CHEMISTRY 101 Calculating Heat Capacity of a Bomb Calorimeter YouTube Average Heat Capacity Of Calorimeter A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat capacity) is. (use 4.184 j g¯ 1 °c¯ 1 as the specific heat of water.) solution: 1) heat given up by warm. The combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3. Average Heat Capacity Of Calorimeter.
From www.slideserve.com
PPT Unit 13 Thermochemistry PowerPoint Presentation, free download Average Heat Capacity Of Calorimeter The combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. Calculate the heat capacity of the calorimeter in j/°c. It can analyze the heat exchange between up to 3 objects. (use 4.184 j g¯ 1 °c¯ 1 as the specific heat of water.) solution: In both cases, the amount of heat absorbed or released by the calorimeter. Average Heat Capacity Of Calorimeter.
From chemistrytalk.org
Calorimetry ChemTalk Average Heat Capacity Of Calorimeter A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat capacity) is. The combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. (use 4.184 j g¯ 1 °c¯ 1 as the specific heat of water.) solution: If $\pu{1.25 g}$. Average Heat Capacity Of Calorimeter.
From www.chegg.com
Solved CALORIMETRY HEAT CAPACITY OF A CALORIMETER Average Heat Capacity Of Calorimeter The temperature increase is measured and, along with the known heat capacity of the calorimeter, is used to calculate the energy produced by the. A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat capacity) is. In both cases, the amount of. Average Heat Capacity Of Calorimeter.
From www.chegg.com
Solved Average heat capacity of calorimeter (J/∗C) Average Heat Capacity Of Calorimeter The combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. (use 4.184 j g¯ 1 °c¯ 1 as the specific heat of water.) solution: The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature. It can analyze the heat exchange between up to. Average Heat Capacity Of Calorimeter.
From janiyahabbgates.blogspot.com
Calorimetry Specific Heat Capacity of Metals Lab Report JaniyahabbGates Average Heat Capacity Of Calorimeter Additionally, it can find the. The combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature. It can analyze the heat exchange between up to 3 objects. Heat always flows from high temperature to. Average Heat Capacity Of Calorimeter.
From www.numerade.com
Table 11.2 Heat Capacity and Enthalpy Change Run 1 Run 2 Average Average Heat Capacity Of Calorimeter In both cases, the amount of heat absorbed or released by the calorimeter is equal in magnitude and opposite in sign to the amount of heat produced or consumed by the reaction. (use 4.184 j g¯ 1 °c¯ 1 as the specific heat of water.) solution: Calculate the heat capacity of the calorimeter in j/°c. 1) heat given up by. Average Heat Capacity Of Calorimeter.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec Average Heat Capacity Of Calorimeter The combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. Additionally, it can find the. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature. If $\pu{1.25 g}$ of glucose are burnt in a calorimeter containing $\pu{0.95 kg}$ of. Calculate the heat capacity. Average Heat Capacity Of Calorimeter.
From www.chegg.com
Solved Average heat capacity (C) of calorimeter in J/∘C= Average Heat Capacity Of Calorimeter Calculate the heat capacity of the calorimeter in j/°c. If $\pu{1.25 g}$ of glucose are burnt in a calorimeter containing $\pu{0.95 kg}$ of. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature. Additionally, it can find the. The amount of heat absorbed or released (q). Average Heat Capacity Of Calorimeter.
From www.youtube.com
CHEMISTRY 101 Constant Pressure Calorimetry YouTube Average Heat Capacity Of Calorimeter The calorimetry calculator can help you solve complex calorimetry problems. The amount of heat absorbed or released (q) by the object depends on its mass. It can analyze the heat exchange between up to 3 objects. The combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. Heat always flows from high temperature to low temperature. In both. Average Heat Capacity Of Calorimeter.
From www.youtube.com
050 Calorimetry YouTube Average Heat Capacity Of Calorimeter The calorimetry calculator can help you solve complex calorimetry problems. The temperature increase is measured and, along with the known heat capacity of the calorimeter, is used to calculate the energy produced by the. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature. The amount. Average Heat Capacity Of Calorimeter.
From www.slideserve.com
PPT Unit 13 Thermochemistry PowerPoint Presentation, free download Average Heat Capacity Of Calorimeter The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature. (use 4.184 j g¯ 1 °c¯ 1 as the specific heat of water.) solution: A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements. Average Heat Capacity Of Calorimeter.