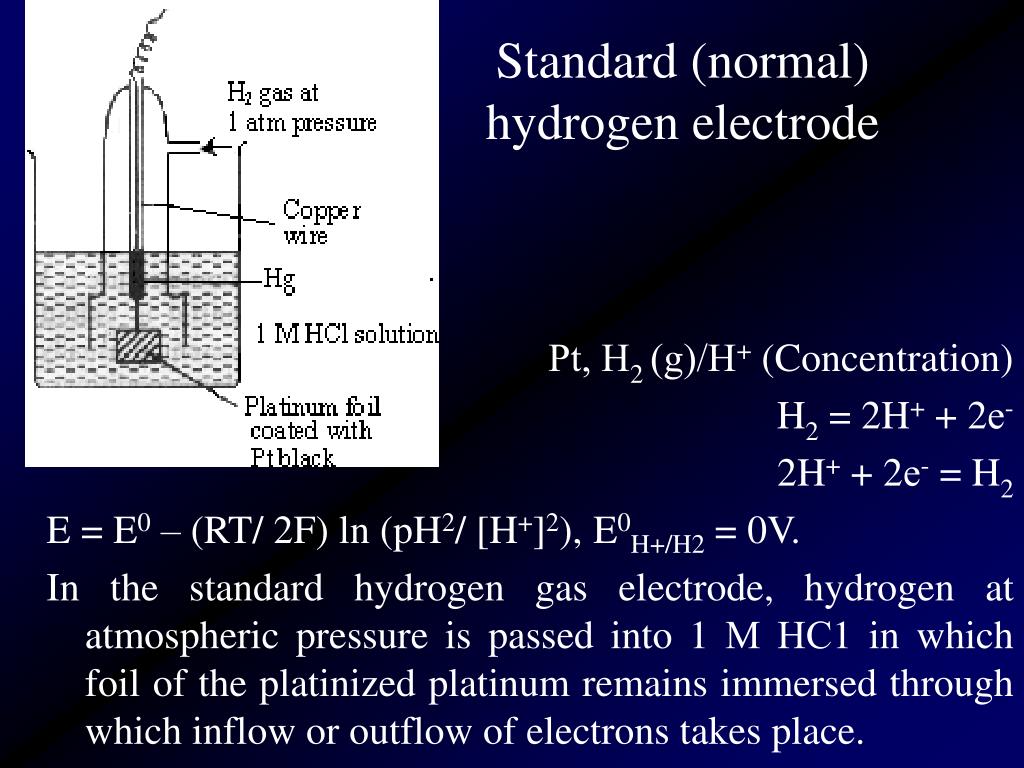
Limitations Of Standard Hydrogen Electrode . Standard hydrogen electrode disadvantages can be summarized as follows: Learn what a standard hydrogen electrode (she) is, how it is constructed, and how it is used as a reference electrode for measuring electrode potential. The standard hydrogen electrode serves as the standard measurement of electrode potential for redox potentials on a thermodynamic scale. The structure of the standard hydrogen electrode is described. Find out the advantages of platinum, the role of hydrogen gas, and the solved example of she. Graphical representation of the onset potentials of the her/hor and oer/orr measured using a. Examples of using the standard hydrogen electrode to determine reduction potentials are given. A standard hydrogen electrode is less reactive than a platinum or nickel electrode,. The standard hydrogen electrode (she) is an excellent reference electrode in electrochemical studies and for determining electrode potentials.
from www.slideserve.com
Learn what a standard hydrogen electrode (she) is, how it is constructed, and how it is used as a reference electrode for measuring electrode potential. Find out the advantages of platinum, the role of hydrogen gas, and the solved example of she. The structure of the standard hydrogen electrode is described. Graphical representation of the onset potentials of the her/hor and oer/orr measured using a. Examples of using the standard hydrogen electrode to determine reduction potentials are given. A standard hydrogen electrode is less reactive than a platinum or nickel electrode,. The standard hydrogen electrode serves as the standard measurement of electrode potential for redox potentials on a thermodynamic scale. The standard hydrogen electrode (she) is an excellent reference electrode in electrochemical studies and for determining electrode potentials. Standard hydrogen electrode disadvantages can be summarized as follows:
PPT LECTURE 7 Electrochemistry. Types of electrodes and their using
Limitations Of Standard Hydrogen Electrode Learn what a standard hydrogen electrode (she) is, how it is constructed, and how it is used as a reference electrode for measuring electrode potential. Learn what a standard hydrogen electrode (she) is, how it is constructed, and how it is used as a reference electrode for measuring electrode potential. The standard hydrogen electrode serves as the standard measurement of electrode potential for redox potentials on a thermodynamic scale. Graphical representation of the onset potentials of the her/hor and oer/orr measured using a. A standard hydrogen electrode is less reactive than a platinum or nickel electrode,. Find out the advantages of platinum, the role of hydrogen gas, and the solved example of she. The standard hydrogen electrode (she) is an excellent reference electrode in electrochemical studies and for determining electrode potentials. Examples of using the standard hydrogen electrode to determine reduction potentials are given. The structure of the standard hydrogen electrode is described. Standard hydrogen electrode disadvantages can be summarized as follows:
From www.pinterest.com
Standard Hydrogen Electrode in 2020 Electron configuration, Ideal Limitations Of Standard Hydrogen Electrode The standard hydrogen electrode (she) is an excellent reference electrode in electrochemical studies and for determining electrode potentials. Graphical representation of the onset potentials of the her/hor and oer/orr measured using a. The standard hydrogen electrode serves as the standard measurement of electrode potential for redox potentials on a thermodynamic scale. Learn what a standard hydrogen electrode (she) is, how. Limitations Of Standard Hydrogen Electrode.
From dohsdocfeco.blob.core.windows.net
Facts About Standard Hydrogen Electrode at Troy Williams blog Limitations Of Standard Hydrogen Electrode Examples of using the standard hydrogen electrode to determine reduction potentials are given. Learn what a standard hydrogen electrode (she) is, how it is constructed, and how it is used as a reference electrode for measuring electrode potential. Graphical representation of the onset potentials of the her/hor and oer/orr measured using a. The standard hydrogen electrode serves as the standard. Limitations Of Standard Hydrogen Electrode.
From www.youtube.com
19.1 Standard hydrogen electrode (HL) YouTube Limitations Of Standard Hydrogen Electrode Learn what a standard hydrogen electrode (she) is, how it is constructed, and how it is used as a reference electrode for measuring electrode potential. Find out the advantages of platinum, the role of hydrogen gas, and the solved example of she. The standard hydrogen electrode (she) is an excellent reference electrode in electrochemical studies and for determining electrode potentials.. Limitations Of Standard Hydrogen Electrode.
From www.slideserve.com
PPT Chapter 20 PowerPoint Presentation, free download ID4435141 Limitations Of Standard Hydrogen Electrode Examples of using the standard hydrogen electrode to determine reduction potentials are given. Graphical representation of the onset potentials of the her/hor and oer/orr measured using a. Learn what a standard hydrogen electrode (she) is, how it is constructed, and how it is used as a reference electrode for measuring electrode potential. Standard hydrogen electrode disadvantages can be summarized as. Limitations Of Standard Hydrogen Electrode.
From www.slideserve.com
PPT Standard hydrogen electrode PowerPoint Presentation, free Limitations Of Standard Hydrogen Electrode The standard hydrogen electrode (she) is an excellent reference electrode in electrochemical studies and for determining electrode potentials. Standard hydrogen electrode disadvantages can be summarized as follows: Learn what a standard hydrogen electrode (she) is, how it is constructed, and how it is used as a reference electrode for measuring electrode potential. A standard hydrogen electrode is less reactive than. Limitations Of Standard Hydrogen Electrode.
From www.studypool.com
SOLUTION Standard hydrogen electrode she or normal Studypool Limitations Of Standard Hydrogen Electrode Examples of using the standard hydrogen electrode to determine reduction potentials are given. The structure of the standard hydrogen electrode is described. Standard hydrogen electrode disadvantages can be summarized as follows: Graphical representation of the onset potentials of the her/hor and oer/orr measured using a. The standard hydrogen electrode (she) is an excellent reference electrode in electrochemical studies and for. Limitations Of Standard Hydrogen Electrode.
From question.pandai.org
Standard Electrode Potential Limitations Of Standard Hydrogen Electrode The standard hydrogen electrode serves as the standard measurement of electrode potential for redox potentials on a thermodynamic scale. A standard hydrogen electrode is less reactive than a platinum or nickel electrode,. Examples of using the standard hydrogen electrode to determine reduction potentials are given. Standard hydrogen electrode disadvantages can be summarized as follows: The structure of the standard hydrogen. Limitations Of Standard Hydrogen Electrode.
From brainly.in
What is standard hydrogen electrode? How is it prepared? Explain with Limitations Of Standard Hydrogen Electrode A standard hydrogen electrode is less reactive than a platinum or nickel electrode,. Find out the advantages of platinum, the role of hydrogen gas, and the solved example of she. The structure of the standard hydrogen electrode is described. Examples of using the standard hydrogen electrode to determine reduction potentials are given. The standard hydrogen electrode serves as the standard. Limitations Of Standard Hydrogen Electrode.
From twitter.com
Digital Kemistry Free Online Chemistry Learning on Twitter "Do you Limitations Of Standard Hydrogen Electrode Learn what a standard hydrogen electrode (she) is, how it is constructed, and how it is used as a reference electrode for measuring electrode potential. A standard hydrogen electrode is less reactive than a platinum or nickel electrode,. Find out the advantages of platinum, the role of hydrogen gas, and the solved example of she. Standard hydrogen electrode disadvantages can. Limitations Of Standard Hydrogen Electrode.
From www.youtube.com
Electrochemistry 06 Standard hydrogen electrode YouTube Limitations Of Standard Hydrogen Electrode The standard hydrogen electrode (she) is an excellent reference electrode in electrochemical studies and for determining electrode potentials. The structure of the standard hydrogen electrode is described. Find out the advantages of platinum, the role of hydrogen gas, and the solved example of she. Learn what a standard hydrogen electrode (she) is, how it is constructed, and how it is. Limitations Of Standard Hydrogen Electrode.
From loegddcbq.blob.core.windows.net
Standard Hydrogen Electrode In English at Janice Wagner blog Limitations Of Standard Hydrogen Electrode Learn what a standard hydrogen electrode (she) is, how it is constructed, and how it is used as a reference electrode for measuring electrode potential. A standard hydrogen electrode is less reactive than a platinum or nickel electrode,. The standard hydrogen electrode (she) is an excellent reference electrode in electrochemical studies and for determining electrode potentials. Graphical representation of the. Limitations Of Standard Hydrogen Electrode.
From www.slideserve.com
PPT Chapter 20 Electrochemistry PowerPoint Presentation, free Limitations Of Standard Hydrogen Electrode A standard hydrogen electrode is less reactive than a platinum or nickel electrode,. Standard hydrogen electrode disadvantages can be summarized as follows: Learn what a standard hydrogen electrode (she) is, how it is constructed, and how it is used as a reference electrode for measuring electrode potential. Graphical representation of the onset potentials of the her/hor and oer/orr measured using. Limitations Of Standard Hydrogen Electrode.
From www.youtube.com
Define standard hydrogen electrode SHE and write the reactions that Limitations Of Standard Hydrogen Electrode Standard hydrogen electrode disadvantages can be summarized as follows: The standard hydrogen electrode serves as the standard measurement of electrode potential for redox potentials on a thermodynamic scale. A standard hydrogen electrode is less reactive than a platinum or nickel electrode,. Find out the advantages of platinum, the role of hydrogen gas, and the solved example of she. Examples of. Limitations Of Standard Hydrogen Electrode.
From www.savemyexams.com
Standard Electrode Potential Edexcel International A Level Chemistry Limitations Of Standard Hydrogen Electrode Examples of using the standard hydrogen electrode to determine reduction potentials are given. A standard hydrogen electrode is less reactive than a platinum or nickel electrode,. Learn what a standard hydrogen electrode (she) is, how it is constructed, and how it is used as a reference electrode for measuring electrode potential. Graphical representation of the onset potentials of the her/hor. Limitations Of Standard Hydrogen Electrode.
From www.studypool.com
SOLUTION Standard hydrogen electrode she or normal Studypool Limitations Of Standard Hydrogen Electrode The structure of the standard hydrogen electrode is described. Examples of using the standard hydrogen electrode to determine reduction potentials are given. The standard hydrogen electrode serves as the standard measurement of electrode potential for redox potentials on a thermodynamic scale. Find out the advantages of platinum, the role of hydrogen gas, and the solved example of she. Graphical representation. Limitations Of Standard Hydrogen Electrode.
From www.slideserve.com
PPT Standard hydrogen electrode PowerPoint Presentation, free Limitations Of Standard Hydrogen Electrode Examples of using the standard hydrogen electrode to determine reduction potentials are given. The structure of the standard hydrogen electrode is described. Standard hydrogen electrode disadvantages can be summarized as follows: The standard hydrogen electrode serves as the standard measurement of electrode potential for redox potentials on a thermodynamic scale. A standard hydrogen electrode is less reactive than a platinum. Limitations Of Standard Hydrogen Electrode.
From www.youtube.com
Standard hydrogen Electrode, Normal hydrogen Electrode Limitations Of Standard Hydrogen Electrode The standard hydrogen electrode (she) is an excellent reference electrode in electrochemical studies and for determining electrode potentials. Graphical representation of the onset potentials of the her/hor and oer/orr measured using a. The structure of the standard hydrogen electrode is described. Standard hydrogen electrode disadvantages can be summarized as follows: Learn what a standard hydrogen electrode (she) is, how it. Limitations Of Standard Hydrogen Electrode.
From loegddcbq.blob.core.windows.net
Standard Hydrogen Electrode In English at Janice Wagner blog Limitations Of Standard Hydrogen Electrode Find out the advantages of platinum, the role of hydrogen gas, and the solved example of she. A standard hydrogen electrode is less reactive than a platinum or nickel electrode,. Standard hydrogen electrode disadvantages can be summarized as follows: The standard hydrogen electrode serves as the standard measurement of electrode potential for redox potentials on a thermodynamic scale. The standard. Limitations Of Standard Hydrogen Electrode.
From issuu.com
standard hydrogen electrode by norainihamzah9 Issuu Limitations Of Standard Hydrogen Electrode A standard hydrogen electrode is less reactive than a platinum or nickel electrode,. The structure of the standard hydrogen electrode is described. The standard hydrogen electrode (she) is an excellent reference electrode in electrochemical studies and for determining electrode potentials. Find out the advantages of platinum, the role of hydrogen gas, and the solved example of she. Graphical representation of. Limitations Of Standard Hydrogen Electrode.
From fdocuments.in
Limitations of Standard Hydrogen Electrode [DOC Document] Limitations Of Standard Hydrogen Electrode Graphical representation of the onset potentials of the her/hor and oer/orr measured using a. The structure of the standard hydrogen electrode is described. A standard hydrogen electrode is less reactive than a platinum or nickel electrode,. The standard hydrogen electrode serves as the standard measurement of electrode potential for redox potentials on a thermodynamic scale. The standard hydrogen electrode (she). Limitations Of Standard Hydrogen Electrode.
From dohsdocfeco.blob.core.windows.net
Facts About Standard Hydrogen Electrode at Troy Williams blog Limitations Of Standard Hydrogen Electrode Standard hydrogen electrode disadvantages can be summarized as follows: The standard hydrogen electrode (she) is an excellent reference electrode in electrochemical studies and for determining electrode potentials. Find out the advantages of platinum, the role of hydrogen gas, and the solved example of she. Learn what a standard hydrogen electrode (she) is, how it is constructed, and how it is. Limitations Of Standard Hydrogen Electrode.
From www.youtube.com
Standard Hydrogen Electrode (SHE)/ Primary reference electrode Gas Limitations Of Standard Hydrogen Electrode Learn what a standard hydrogen electrode (she) is, how it is constructed, and how it is used as a reference electrode for measuring electrode potential. The standard hydrogen electrode serves as the standard measurement of electrode potential for redox potentials on a thermodynamic scale. Find out the advantages of platinum, the role of hydrogen gas, and the solved example of. Limitations Of Standard Hydrogen Electrode.
From www.slideserve.com
PPT Standard Reference Electrode Standard Hydrogen Electrode (SHE Limitations Of Standard Hydrogen Electrode The structure of the standard hydrogen electrode is described. Find out the advantages of platinum, the role of hydrogen gas, and the solved example of she. Standard hydrogen electrode disadvantages can be summarized as follows: A standard hydrogen electrode is less reactive than a platinum or nickel electrode,. The standard hydrogen electrode serves as the standard measurement of electrode potential. Limitations Of Standard Hydrogen Electrode.
From pubs.acs.org
Standard and Reversible Hydrogen Electrodes Theory, Design, Operation Limitations Of Standard Hydrogen Electrode Examples of using the standard hydrogen electrode to determine reduction potentials are given. Standard hydrogen electrode disadvantages can be summarized as follows: The structure of the standard hydrogen electrode is described. The standard hydrogen electrode serves as the standard measurement of electrode potential for redox potentials on a thermodynamic scale. Learn what a standard hydrogen electrode (she) is, how it. Limitations Of Standard Hydrogen Electrode.
From brainly.in
Draw a neat and labelled diagram of standard hydrogen electrode Limitations Of Standard Hydrogen Electrode Learn what a standard hydrogen electrode (she) is, how it is constructed, and how it is used as a reference electrode for measuring electrode potential. A standard hydrogen electrode is less reactive than a platinum or nickel electrode,. The structure of the standard hydrogen electrode is described. Graphical representation of the onset potentials of the her/hor and oer/orr measured using. Limitations Of Standard Hydrogen Electrode.
From byjus.com
What is standard hydrogen electrode? Limitations Of Standard Hydrogen Electrode Standard hydrogen electrode disadvantages can be summarized as follows: Learn what a standard hydrogen electrode (she) is, how it is constructed, and how it is used as a reference electrode for measuring electrode potential. A standard hydrogen electrode is less reactive than a platinum or nickel electrode,. The structure of the standard hydrogen electrode is described. The standard hydrogen electrode. Limitations Of Standard Hydrogen Electrode.
From www.shutterstock.com
Diagramme standard d'électrode à hydrogène. Illustration image Limitations Of Standard Hydrogen Electrode The structure of the standard hydrogen electrode is described. A standard hydrogen electrode is less reactive than a platinum or nickel electrode,. Graphical representation of the onset potentials of the her/hor and oer/orr measured using a. The standard hydrogen electrode (she) is an excellent reference electrode in electrochemical studies and for determining electrode potentials. Standard hydrogen electrode disadvantages can be. Limitations Of Standard Hydrogen Electrode.
From www.slideserve.com
PPT LECTURE 7 Electrochemistry. Types of electrodes and their using Limitations Of Standard Hydrogen Electrode The structure of the standard hydrogen electrode is described. A standard hydrogen electrode is less reactive than a platinum or nickel electrode,. The standard hydrogen electrode (she) is an excellent reference electrode in electrochemical studies and for determining electrode potentials. The standard hydrogen electrode serves as the standard measurement of electrode potential for redox potentials on a thermodynamic scale. Graphical. Limitations Of Standard Hydrogen Electrode.
From byjus.com
Standard Hydrogen Electrode Definition, Construction, and Labelled Limitations Of Standard Hydrogen Electrode Standard hydrogen electrode disadvantages can be summarized as follows: The structure of the standard hydrogen electrode is described. Find out the advantages of platinum, the role of hydrogen gas, and the solved example of she. A standard hydrogen electrode is less reactive than a platinum or nickel electrode,. The standard hydrogen electrode (she) is an excellent reference electrode in electrochemical. Limitations Of Standard Hydrogen Electrode.
From www.slideserve.com
PPT Chapter 20 Electrochemistry PowerPoint Presentation, free Limitations Of Standard Hydrogen Electrode The standard hydrogen electrode serves as the standard measurement of electrode potential for redox potentials on a thermodynamic scale. Learn what a standard hydrogen electrode (she) is, how it is constructed, and how it is used as a reference electrode for measuring electrode potential. The standard hydrogen electrode (she) is an excellent reference electrode in electrochemical studies and for determining. Limitations Of Standard Hydrogen Electrode.
From www.youtube.com
standard hydrogen electrode (with examples) YouTube Limitations Of Standard Hydrogen Electrode Standard hydrogen electrode disadvantages can be summarized as follows: The standard hydrogen electrode (she) is an excellent reference electrode in electrochemical studies and for determining electrode potentials. The standard hydrogen electrode serves as the standard measurement of electrode potential for redox potentials on a thermodynamic scale. A standard hydrogen electrode is less reactive than a platinum or nickel electrode,. Learn. Limitations Of Standard Hydrogen Electrode.
From www.youtube.com
Standard Hydrogen Electrode Demonstration YouTube Limitations Of Standard Hydrogen Electrode Find out the advantages of platinum, the role of hydrogen gas, and the solved example of she. Graphical representation of the onset potentials of the her/hor and oer/orr measured using a. A standard hydrogen electrode is less reactive than a platinum or nickel electrode,. Learn what a standard hydrogen electrode (she) is, how it is constructed, and how it is. Limitations Of Standard Hydrogen Electrode.
From dokumen.tips
(DOC) Limitations of Standard Hydrogen Electrode DOKUMEN.TIPS Limitations Of Standard Hydrogen Electrode The structure of the standard hydrogen electrode is described. Learn what a standard hydrogen electrode (she) is, how it is constructed, and how it is used as a reference electrode for measuring electrode potential. Graphical representation of the onset potentials of the her/hor and oer/orr measured using a. Find out the advantages of platinum, the role of hydrogen gas, and. Limitations Of Standard Hydrogen Electrode.
From pubs.acs.org
Standard and Reversible Hydrogen Electrodes Theory, Design, Operation Limitations Of Standard Hydrogen Electrode Examples of using the standard hydrogen electrode to determine reduction potentials are given. The structure of the standard hydrogen electrode is described. Graphical representation of the onset potentials of the her/hor and oer/orr measured using a. Standard hydrogen electrode disadvantages can be summarized as follows: Find out the advantages of platinum, the role of hydrogen gas, and the solved example. Limitations Of Standard Hydrogen Electrode.
From chemistnotes.com
Standard hydrogen electrode(SHE) Definition, diagram, application Limitations Of Standard Hydrogen Electrode Standard hydrogen electrode disadvantages can be summarized as follows: A standard hydrogen electrode is less reactive than a platinum or nickel electrode,. Learn what a standard hydrogen electrode (she) is, how it is constructed, and how it is used as a reference electrode for measuring electrode potential. The standard hydrogen electrode serves as the standard measurement of electrode potential for. Limitations Of Standard Hydrogen Electrode.