Titration Of Hf With Naoh . The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. The volume of hcl needed to reach the equivalence point is \[v_{e. Includes kit list and safety instructions. The reaction of the weak acid, acetic acid, with a. Multiply the molarity of the strong base naoh by the volume of. To see how this data can be used, follow the titration of hydrofluoric acid against sodium hydroxide below. Consider the titration of \(\ce{hcl}\) with \(\ce{naoh}\), that is,. Relating titrations to stoichiometry and limiting reagent problems. Dip probe of digital ph meter into mixed unknown solution. Construct a titration curve for the titration of 25.0 ml of 0.125 m naoh with 0.0625 m hcl. Make 0.2n sodium hydroxide (naoh) solution. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Hydrofluoric acid, #hf#, a weak acid, will react with sodium hydroxide, #naoh#, a strong base, to produce aqueous sodium. Hf (aq) + naoh (aq) h2o (l) +.
from www.numerade.com
The volume of hcl needed to reach the equivalence point is \[v_{e. Hydrofluoric acid, #hf#, a weak acid, will react with sodium hydroxide, #naoh#, a strong base, to produce aqueous sodium. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Multiply the molarity of the strong base naoh by the volume of. To see how this data can be used, follow the titration of hydrofluoric acid against sodium hydroxide below. Dip probe of digital ph meter into mixed unknown solution. Construct a titration curve for the titration of 25.0 ml of 0.125 m naoh with 0.0625 m hcl. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Relating titrations to stoichiometry and limiting reagent problems. The reaction of the weak acid, acetic acid, with a.
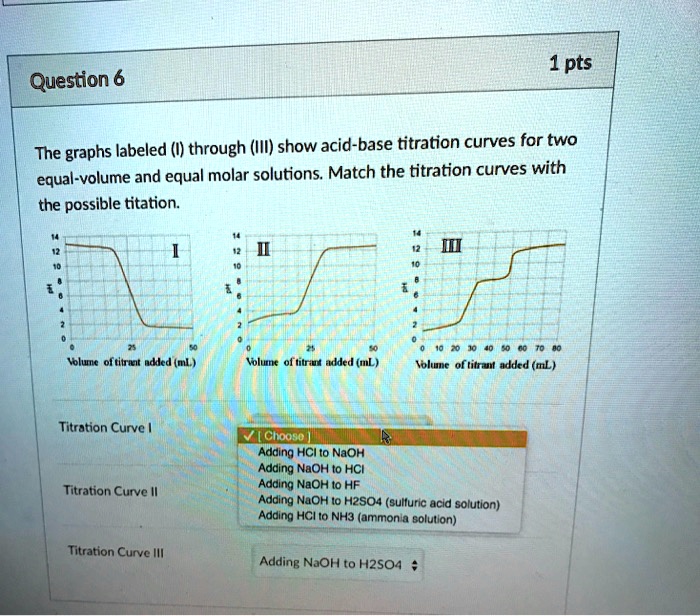
SOLVED Question 6 The graphs labeled (I) through (III) show acidbase
Titration Of Hf With Naoh Dip probe of digital ph meter into mixed unknown solution. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Relating titrations to stoichiometry and limiting reagent problems. Construct a titration curve for the titration of 25.0 ml of 0.125 m naoh with 0.0625 m hcl. Dip probe of digital ph meter into mixed unknown solution. Make 0.2n sodium hydroxide (naoh) solution. Multiply the molarity of the strong base naoh by the volume of. The volume of hcl needed to reach the equivalence point is \[v_{e. Hydrofluoric acid, #hf#, a weak acid, will react with sodium hydroxide, #naoh#, a strong base, to produce aqueous sodium. Hf (aq) + naoh (aq) h2o (l) +. The reaction of the weak acid, acetic acid, with a. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Includes kit list and safety instructions. To see how this data can be used, follow the titration of hydrofluoric acid against sodium hydroxide below. Consider the titration of \(\ce{hcl}\) with \(\ce{naoh}\), that is,.
From www.chegg.com
A titration curve is shown below for a solution of Titration Of Hf With Naoh Hf (aq) + naoh (aq) h2o (l) +. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Hydrofluoric acid, #hf#, a weak acid, will react with sodium hydroxide, #naoh#, a strong base, to produce aqueous sodium. Relating titrations to stoichiometry and limiting reagent problems. Dip. Titration Of Hf With Naoh.
From www.bartleby.com
Answered Consider the following indicators… bartleby Titration Of Hf With Naoh Hf (aq) + naoh (aq) h2o (l) +. Consider the titration of \(\ce{hcl}\) with \(\ce{naoh}\), that is,. Hydrofluoric acid, #hf#, a weak acid, will react with sodium hydroxide, #naoh#, a strong base, to produce aqueous sodium. The reaction of the weak acid, acetic acid, with a. Construct a titration curve for the titration of 25.0 ml of 0.125 m naoh. Titration Of Hf With Naoh.
From www.vrogue.co
Top 10 Apparatus For Titration Of 2020 No Place Calle vrogue.co Titration Of Hf With Naoh Consider the titration of \(\ce{hcl}\) with \(\ce{naoh}\), that is,. Hydrofluoric acid, #hf#, a weak acid, will react with sodium hydroxide, #naoh#, a strong base, to produce aqueous sodium. Make 0.2n sodium hydroxide (naoh) solution. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. The volume of hcl needed to reach the. Titration Of Hf With Naoh.
From mavink.com
H2so4 Titration Curve Titration Of Hf With Naoh Make 0.2n sodium hydroxide (naoh) solution. Includes kit list and safety instructions. Multiply the molarity of the strong base naoh by the volume of. The volume of hcl needed to reach the equivalence point is \[v_{e. To see how this data can be used, follow the titration of hydrofluoric acid against sodium hydroxide below. Relating titrations to stoichiometry and limiting. Titration Of Hf With Naoh.
From www.numerade.com
SOLVED Consider the titration of 25.00 mL of 0.100 M hydrofluoric acid Titration Of Hf With Naoh Hydrofluoric acid, #hf#, a weak acid, will react with sodium hydroxide, #naoh#, a strong base, to produce aqueous sodium. Make 0.2n sodium hydroxide (naoh) solution. The reaction of the weak acid, acetic acid, with a. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Multiply. Titration Of Hf With Naoh.
From plotly.com
KHP and NaOH Titration Curve line chart made by Kylclk plotly Titration Of Hf With Naoh Make 0.2n sodium hydroxide (naoh) solution. Dip probe of digital ph meter into mixed unknown solution. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. To see how this data can be used, follow the titration of hydrofluoric acid against sodium hydroxide below. Multiply the. Titration Of Hf With Naoh.
From fyobaweqw.blob.core.windows.net
Indicators Titration Define at James Honeycutt blog Titration Of Hf With Naoh Includes kit list and safety instructions. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Make 0.2n sodium hydroxide (naoh) solution. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. The volume of hcl needed to. Titration Of Hf With Naoh.
From www.chegg.com
Solved For The Titration Of HF With NaOH, Which Location Titration Of Hf With Naoh Multiply the molarity of the strong base naoh by the volume of. The volume of hcl needed to reach the equivalence point is \[v_{e. The reaction of the weak acid, acetic acid, with a. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Construct a. Titration Of Hf With Naoh.
From www.chegg.com
Solved HF(aq)+OHH(aq) F(aq)+H20( A student performed a Titration Of Hf With Naoh Consider the titration of \(\ce{hcl}\) with \(\ce{naoh}\), that is,. Includes kit list and safety instructions. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Hf (aq) + naoh (aq) h2o (l) +. The reaction of the weak acid, acetic acid, with a. Make 0.2n sodium. Titration Of Hf With Naoh.
From www.youtube.com
Conductometric titration I strong acid (HCl) versus strong base Titration Of Hf With Naoh Hf (aq) + naoh (aq) h2o (l) +. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Make 0.2n sodium hydroxide (naoh) solution. Construct a titration curve for the titration of 25.0 ml of 0.125 m naoh with 0.0625 m hcl. Hydrofluoric acid, #hf#, a. Titration Of Hf With Naoh.
From solvedlib.com
A titration of 59.00 ml of NaOH with 0.1000 M HCl was… SolvedLib Titration Of Hf With Naoh The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Construct a titration curve for the titration of 25.0 ml of 0.125 m naoh with 0.0625 m hcl. To see how this data can be used, follow the titration of hydrofluoric acid against sodium hydroxide below.. Titration Of Hf With Naoh.
From www.numerade.com
A student performed a titration of HF(aq) with NaOH(aq). The net ionic Titration Of Hf With Naoh Multiply the molarity of the strong base naoh by the volume of. Dip probe of digital ph meter into mixed unknown solution. The volume of hcl needed to reach the equivalence point is \[v_{e. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Make 0.2n sodium hydroxide (naoh) solution. To see. Titration Of Hf With Naoh.
From mavink.com
Titration Reaction Titration Of Hf With Naoh The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Hf (aq) + naoh (aq) h2o (l) +. Make 0.2n sodium hydroxide (naoh) solution. Relating titrations to stoichiometry and limiting reagent problems. Construct a titration curve for the titration of 25.0 ml of 0.125 m naoh. Titration Of Hf With Naoh.
From www.youtube.com
Titration of a monoprotic strong acid (HCl) and monoprotic strong base Titration Of Hf With Naoh Dip probe of digital ph meter into mixed unknown solution. The reaction of the weak acid, acetic acid, with a. Multiply the molarity of the strong base naoh by the volume of. Hf (aq) + naoh (aq) h2o (l) +. Construct a titration curve for the titration of 25.0 ml of 0.125 m naoh with 0.0625 m hcl. To see. Titration Of Hf With Naoh.
From www.chegg.com
Solved Below is a graph of pH vs. volume of NaOH(mL) added Titration Of Hf With Naoh Consider the titration of \(\ce{hcl}\) with \(\ce{naoh}\), that is,. The reaction of the weak acid, acetic acid, with a. Make 0.2n sodium hydroxide (naoh) solution. Construct a titration curve for the titration of 25.0 ml of 0.125 m naoh with 0.0625 m hcl. Hydrofluoric acid, #hf#, a weak acid, will react with sodium hydroxide, #naoh#, a strong base, to produce. Titration Of Hf With Naoh.
From schoolbag.info
What volume of NaOH( aq ) would be needed to reach the equivalence Titration Of Hf With Naoh The volume of hcl needed to reach the equivalence point is \[v_{e. Dip probe of digital ph meter into mixed unknown solution. Includes kit list and safety instructions. To see how this data can be used, follow the titration of hydrofluoric acid against sodium hydroxide below. Consider the titration of \(\ce{hcl}\) with \(\ce{naoh}\), that is,. Make 0.2n sodium hydroxide (naoh). Titration Of Hf With Naoh.
From www.numerade.com
SOLVED For the titration of HF with NaOH; which location on the Titration Of Hf With Naoh Hf (aq) + naoh (aq) h2o (l) +. Hydrofluoric acid, #hf#, a weak acid, will react with sodium hydroxide, #naoh#, a strong base, to produce aqueous sodium. To see how this data can be used, follow the titration of hydrofluoric acid against sodium hydroxide below. Construct a titration curve for the titration of 25.0 ml of 0.125 m naoh with. Titration Of Hf With Naoh.
From www.vrogue.co
Solved The Titration Curve Shown Below Is For The Tit vrogue.co Titration Of Hf With Naoh Includes kit list and safety instructions. Hydrofluoric acid, #hf#, a weak acid, will react with sodium hydroxide, #naoh#, a strong base, to produce aqueous sodium. The reaction of the weak acid, acetic acid, with a. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Consider the titration of \(\ce{hcl}\) with \(\ce{naoh}\),. Titration Of Hf With Naoh.
From www.chegg.com
Solved What is the average concentration of your Titration Of Hf With Naoh Dip probe of digital ph meter into mixed unknown solution. Hydrofluoric acid, #hf#, a weak acid, will react with sodium hydroxide, #naoh#, a strong base, to produce aqueous sodium. Consider the titration of \(\ce{hcl}\) with \(\ce{naoh}\), that is,. Construct a titration curve for the titration of 25.0 ml of 0.125 m naoh with 0.0625 m hcl. To see how this. Titration Of Hf With Naoh.
From www.numerade.com
SOLVED 'Which of the following titrations result in a basic solution Titration Of Hf With Naoh Relating titrations to stoichiometry and limiting reagent problems. To see how this data can be used, follow the titration of hydrofluoric acid against sodium hydroxide below. Construct a titration curve for the titration of 25.0 ml of 0.125 m naoh with 0.0625 m hcl. Multiply the molarity of the strong base naoh by the volume of. The reaction of the. Titration Of Hf With Naoh.
From www.numerade.com
SOLVED A 50.0 mL sample of 0.100 M HF solution is titrated with 0.125 Titration Of Hf With Naoh Consider the titration of \(\ce{hcl}\) with \(\ce{naoh}\), that is,. Multiply the molarity of the strong base naoh by the volume of. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Make 0.2n sodium hydroxide (naoh) solution. Relating titrations to stoichiometry and limiting reagent problems. Hf (aq) + naoh (aq) h2o (l). Titration Of Hf With Naoh.
From gioilucnh.blob.core.windows.net
Titration Titrant Equivalence Point at Pamella blog Titration Of Hf With Naoh To see how this data can be used, follow the titration of hydrofluoric acid against sodium hydroxide below. The reaction of the weak acid, acetic acid, with a. Multiply the molarity of the strong base naoh by the volume of. Make 0.2n sodium hydroxide (naoh) solution. The titration of a weak acid with a strong base involves the direct transfer. Titration Of Hf With Naoh.
From www.youtube.com
Titration Weak Acid Strong Base Equivalence Point YouTube Titration Of Hf With Naoh Make 0.2n sodium hydroxide (naoh) solution. To see how this data can be used, follow the titration of hydrofluoric acid against sodium hydroxide below. The reaction of the weak acid, acetic acid, with a. Includes kit list and safety instructions. The volume of hcl needed to reach the equivalence point is \[v_{e. Dip probe of digital ph meter into mixed. Titration Of Hf With Naoh.
From www.numerade.com
SOLVED 4) (4 pts) In lab you titrated HCl with NaOH Sketch the Titration Of Hf With Naoh The reaction of the weak acid, acetic acid, with a. Relating titrations to stoichiometry and limiting reagent problems. Multiply the molarity of the strong base naoh by the volume of. To see how this data can be used, follow the titration of hydrofluoric acid against sodium hydroxide below. Hf (aq) + naoh (aq) h2o (l) +. Consider the titration of. Titration Of Hf With Naoh.
From www.researchgate.net
Titration of 1 mL diluted bath (H2SO4/H3PO4) with 0.5 M NaOH in water Titration Of Hf With Naoh Includes kit list and safety instructions. Dip probe of digital ph meter into mixed unknown solution. Hf (aq) + naoh (aq) h2o (l) +. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Multiply the molarity of the strong base naoh by the volume of. Hydrofluoric acid, #hf#, a weak acid,. Titration Of Hf With Naoh.
From www.researchgate.net
Titration, by various bases and in various solvents, of the acids HNO 3 Titration Of Hf With Naoh The volume of hcl needed to reach the equivalence point is \[v_{e. Hf (aq) + naoh (aq) h2o (l) +. Consider the titration of \(\ce{hcl}\) with \(\ce{naoh}\), that is,. To see how this data can be used, follow the titration of hydrofluoric acid against sodium hydroxide below. Relating titrations to stoichiometry and limiting reagent problems. Construct a titration curve for. Titration Of Hf With Naoh.
From www.chegg.com
Solved For the titration of HF with NaOH, which location on Titration Of Hf With Naoh Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Includes kit list and safety instructions. Consider the titration of \(\ce{hcl}\) with \(\ce{naoh}\), that is,. To see how this data can be used, follow the titration of hydrofluoric acid against sodium hydroxide below. Dip probe of digital ph meter into mixed unknown. Titration Of Hf With Naoh.
From www.slideserve.com
PPT TITRATION PowerPoint Presentation, free download ID1459481 Titration Of Hf With Naoh Consider the titration of \(\ce{hcl}\) with \(\ce{naoh}\), that is,. Hf (aq) + naoh (aq) h2o (l) +. Multiply the molarity of the strong base naoh by the volume of. The reaction of the weak acid, acetic acid, with a. Construct a titration curve for the titration of 25.0 ml of 0.125 m naoh with 0.0625 m hcl. Make 0.2n sodium. Titration Of Hf With Naoh.
From www.numerade.com
SOLVED Question 6 The graphs labeled (I) through (III) show acidbase Titration Of Hf With Naoh Hydrofluoric acid, #hf#, a weak acid, will react with sodium hydroxide, #naoh#, a strong base, to produce aqueous sodium. Includes kit list and safety instructions. To see how this data can be used, follow the titration of hydrofluoric acid against sodium hydroxide below. Relating titrations to stoichiometry and limiting reagent problems. Make 0.2n sodium hydroxide (naoh) solution. Construct a titration. Titration Of Hf With Naoh.
From pubs.sciepub.com
Figure 5B. Plot of the titration of strong acid (HCl= 0.1M) with strong Titration Of Hf With Naoh Construct a titration curve for the titration of 25.0 ml of 0.125 m naoh with 0.0625 m hcl. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Consider the titration of \(\ce{hcl}\) with \(\ce{naoh}\), that is,. The reaction of the weak acid, acetic acid, with. Titration Of Hf With Naoh.
From fyoltrxao.blob.core.windows.net
The Titration Equation Is at Lana Tracy blog Titration Of Hf With Naoh Dip probe of digital ph meter into mixed unknown solution. Hydrofluoric acid, #hf#, a weak acid, will react with sodium hydroxide, #naoh#, a strong base, to produce aqueous sodium. Hf (aq) + naoh (aq) h2o (l) +. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Consider the titration of \(\ce{hcl}\). Titration Of Hf With Naoh.
From hxeptiewa.blob.core.windows.net
Find Equivalence Point From Titration at Roger Cornelius blog Titration Of Hf With Naoh Consider the titration of \(\ce{hcl}\) with \(\ce{naoh}\), that is,. Dip probe of digital ph meter into mixed unknown solution. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Construct a titration curve for the titration of 25.0 ml of 0.125 m naoh with 0.0625 m hcl. Hydrofluoric acid, #hf#, a weak. Titration Of Hf With Naoh.
From www.youtube.com
Titration Of NaOH with Oxalic Acid ( Class XI, Practical1) YouTube Titration Of Hf With Naoh Consider the titration of \(\ce{hcl}\) with \(\ce{naoh}\), that is,. Relating titrations to stoichiometry and limiting reagent problems. Hydrofluoric acid, #hf#, a weak acid, will react with sodium hydroxide, #naoh#, a strong base, to produce aqueous sodium. Includes kit list and safety instructions. Hf (aq) + naoh (aq) h2o (l) +. The volume of hcl needed to reach the equivalence point. Titration Of Hf With Naoh.
From exoafzput.blob.core.windows.net
Titration Graph Point at Arleen McKoy blog Titration Of Hf With Naoh Dip probe of digital ph meter into mixed unknown solution. Includes kit list and safety instructions. Relating titrations to stoichiometry and limiting reagent problems. Hydrofluoric acid, #hf#, a weak acid, will react with sodium hydroxide, #naoh#, a strong base, to produce aqueous sodium. Make 0.2n sodium hydroxide (naoh) solution. The volume of hcl needed to reach the equivalence point is. Titration Of Hf With Naoh.
From www.coursehero.com
[Solved] Answer the following questions related to HF and the titration Titration Of Hf With Naoh Includes kit list and safety instructions. The volume of hcl needed to reach the equivalence point is \[v_{e. The reaction of the weak acid, acetic acid, with a. Relating titrations to stoichiometry and limiting reagent problems. Dip probe of digital ph meter into mixed unknown solution. The titration of a weak acid with a strong base involves the direct transfer. Titration Of Hf With Naoh.