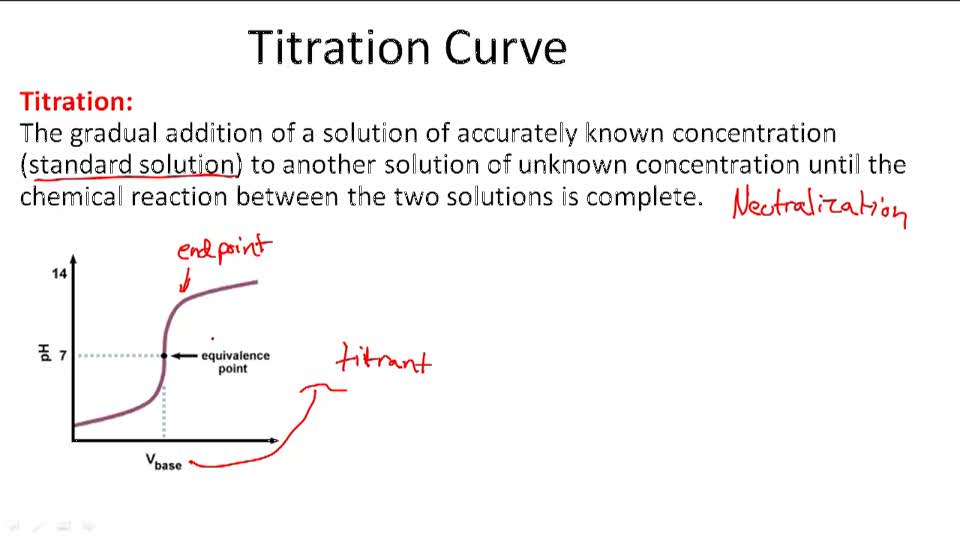
How To Calculate Concentration Using Titration . Concordant titres should be used when. Data collected during a titration allows chemists to determine a solution’s unknown concentration. They can also be used to calculate the ph after a given point during a titration. Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved in a sample of known concentration. The former quantity could be obtained via a stoichiometric ratio from the. To calculate concentration, we need to know the amount of naoh and the volume of solution in which it is dissolved. Titration calculations are used to find the concentration of unknown solutions. The process of calculating concentration from titration data is described and illustrated. The concentration of a species in solution can be determined by quantitative analysis. When the reaction between the analyte and titrant. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly. One such method is a titration, in which a measured volume of a solution of one substance, the titrant, is.
from www.ck12.org
To calculate concentration, we need to know the amount of naoh and the volume of solution in which it is dissolved. Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved in a sample of known concentration. The process of calculating concentration from titration data is described and illustrated. The former quantity could be obtained via a stoichiometric ratio from the. They can also be used to calculate the ph after a given point during a titration. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly. Data collected during a titration allows chemists to determine a solution’s unknown concentration. One such method is a titration, in which a measured volume of a solution of one substance, the titrant, is. The concentration of a species in solution can be determined by quantitative analysis. Titration calculations are used to find the concentration of unknown solutions.
Titration (Calculations) Overview ( Video ) Chemistry CK12
How To Calculate Concentration Using Titration One such method is a titration, in which a measured volume of a solution of one substance, the titrant, is. The concentration of a species in solution can be determined by quantitative analysis. Titration calculations are used to find the concentration of unknown solutions. To calculate concentration, we need to know the amount of naoh and the volume of solution in which it is dissolved. The former quantity could be obtained via a stoichiometric ratio from the. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly. Concordant titres should be used when. They can also be used to calculate the ph after a given point during a titration. Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved in a sample of known concentration. The process of calculating concentration from titration data is described and illustrated. One such method is a titration, in which a measured volume of a solution of one substance, the titrant, is. Data collected during a titration allows chemists to determine a solution’s unknown concentration. When the reaction between the analyte and titrant.
From exommzgkk.blob.core.windows.net
Titration Concentration Of Solution at Tony Pelletier blog How To Calculate Concentration Using Titration When the reaction between the analyte and titrant. To calculate concentration, we need to know the amount of naoh and the volume of solution in which it is dissolved. One such method is a titration, in which a measured volume of a solution of one substance, the titrant, is. The former quantity could be obtained via a stoichiometric ratio from. How To Calculate Concentration Using Titration.
From www.youtube.com
A Simple Guide to Titration Calculations Part 1 Concentration YouTube How To Calculate Concentration Using Titration Data collected during a titration allows chemists to determine a solution’s unknown concentration. Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved in a sample of known concentration. To calculate concentration, we need to know the amount of naoh and the volume of solution in which it is dissolved. The former quantity could be. How To Calculate Concentration Using Titration.
From www.slideserve.com
PPT Unit 19 Acid Base Equilibria Titrations PowerPoint Presentation How To Calculate Concentration Using Titration Concordant titres should be used when. The former quantity could be obtained via a stoichiometric ratio from the. Data collected during a titration allows chemists to determine a solution’s unknown concentration. The process of calculating concentration from titration data is described and illustrated. They can also be used to calculate the ph after a given point during a titration. Titration. How To Calculate Concentration Using Titration.
From dxomwdfni.blob.core.windows.net
Titration Ph Formula at Farrah Carleton blog How To Calculate Concentration Using Titration Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved in a sample of known concentration. They can also be used to calculate the ph after a given point during a titration. Concordant titres should be used when. Data collected during a titration allows chemists to determine a solution’s unknown concentration. The former quantity could. How To Calculate Concentration Using Titration.
From www.ck12.org
Titration (Calculations) Overview ( Video ) Chemistry CK12 How To Calculate Concentration Using Titration Concordant titres should be used when. The concentration of a species in solution can be determined by quantitative analysis. The process of calculating concentration from titration data is described and illustrated. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly. They can also be used to calculate the ph after a given point during. How To Calculate Concentration Using Titration.
From loeppodiz.blob.core.windows.net
Titration Calculation Step By Step Pdf at Linda Tibbetts blog How To Calculate Concentration Using Titration The process of calculating concentration from titration data is described and illustrated. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly. Concordant titres should be used when. Titration calculations are used to find the concentration of unknown solutions. To calculate concentration, we need to know the amount of naoh and the volume of solution. How To Calculate Concentration Using Titration.
From www.slideserve.com
PPT Calculating concentrations PowerPoint Presentation, free download How To Calculate Concentration Using Titration Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved in a sample of known concentration. The former quantity could be obtained via a stoichiometric ratio from the. When the reaction between the analyte and titrant. They can also be used to calculate the ph after a given point during a titration. The concentration of. How To Calculate Concentration Using Titration.
From www.youtube.com
Titration of unknown weak acid with strong base YouTube How To Calculate Concentration Using Titration Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved in a sample of known concentration. The concentration of a species in solution can be determined by quantitative analysis. Data collected during a titration allows chemists to determine a solution’s unknown concentration. One such method is a titration, in which a measured volume of a. How To Calculate Concentration Using Titration.
From www.slideserve.com
PPT Calculating concentrations PowerPoint Presentation, free download How To Calculate Concentration Using Titration In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly. To calculate concentration, we need to know the amount of naoh and the volume of solution in which it is dissolved. Data collected during a titration allows chemists to determine a solution’s unknown concentration. One such method is a titration, in which a measured volume. How To Calculate Concentration Using Titration.
From www.reddit.com
How to find concentration from a titration curve? r/chemistryhelp How To Calculate Concentration Using Titration The process of calculating concentration from titration data is described and illustrated. Data collected during a titration allows chemists to determine a solution’s unknown concentration. The former quantity could be obtained via a stoichiometric ratio from the. Concordant titres should be used when. To calculate concentration, we need to know the amount of naoh and the volume of solution in. How To Calculate Concentration Using Titration.
From mungfali.com
Acid Base Titration Procedure How To Calculate Concentration Using Titration The former quantity could be obtained via a stoichiometric ratio from the. When the reaction between the analyte and titrant. Titration calculations are used to find the concentration of unknown solutions. Data collected during a titration allows chemists to determine a solution’s unknown concentration. To calculate concentration, we need to know the amount of naoh and the volume of solution. How To Calculate Concentration Using Titration.
From theedge.com.hk
Chemistry How To Titration The Edge How To Calculate Concentration Using Titration When the reaction between the analyte and titrant. The concentration of a species in solution can be determined by quantitative analysis. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly. Concordant titres should be used when. Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved in a sample. How To Calculate Concentration Using Titration.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog How To Calculate Concentration Using Titration When the reaction between the analyte and titrant. Titration calculations are used to find the concentration of unknown solutions. The former quantity could be obtained via a stoichiometric ratio from the. The concentration of a species in solution can be determined by quantitative analysis. The process of calculating concentration from titration data is described and illustrated. Titration is a method. How To Calculate Concentration Using Titration.
From www.youtube.com
Worked example Determining solute concentration by acidbase titration How To Calculate Concentration Using Titration They can also be used to calculate the ph after a given point during a titration. Titration calculations are used to find the concentration of unknown solutions. When the reaction between the analyte and titrant. The concentration of a species in solution can be determined by quantitative analysis. The process of calculating concentration from titration data is described and illustrated.. How To Calculate Concentration Using Titration.
From www.ck12.org
Titration (Calculations) Example 3 ( Video ) Chemistry CK12 How To Calculate Concentration Using Titration Titration calculations are used to find the concentration of unknown solutions. One such method is a titration, in which a measured volume of a solution of one substance, the titrant, is. The former quantity could be obtained via a stoichiometric ratio from the. They can also be used to calculate the ph after a given point during a titration. The. How To Calculate Concentration Using Titration.
From www.science-revision.co.uk
Titrations How To Calculate Concentration Using Titration Concordant titres should be used when. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly. When the reaction between the analyte and titrant. Titration calculations are used to find the concentration of unknown solutions. The former quantity could be obtained via a stoichiometric ratio from the. Data collected during a titration allows chemists to. How To Calculate Concentration Using Titration.
From www.slideserve.com
PPT Calculating concentrations PowerPoint Presentation, free download How To Calculate Concentration Using Titration Concordant titres should be used when. The former quantity could be obtained via a stoichiometric ratio from the. One such method is a titration, in which a measured volume of a solution of one substance, the titrant, is. Data collected during a titration allows chemists to determine a solution’s unknown concentration. The concentration of a species in solution can be. How To Calculate Concentration Using Titration.
From www.youtube.com
CHEM 101 Titration of Unknown Triprotic Acid YouTube How To Calculate Concentration Using Titration When the reaction between the analyte and titrant. To calculate concentration, we need to know the amount of naoh and the volume of solution in which it is dissolved. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly. One such method is a titration, in which a measured volume of a solution of one. How To Calculate Concentration Using Titration.
From www.wikihow.com
5 Easy Ways to Calculate the Concentration of a Solution How To Calculate Concentration Using Titration In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly. Concordant titres should be used when. One such method is a titration, in which a measured volume of a solution of one substance, the titrant, is. The concentration of a species in solution can be determined by quantitative analysis. The process of calculating concentration from. How To Calculate Concentration Using Titration.
From www.youtube.com
How to Calculate Concentration using Titration Example in Chemistry How To Calculate Concentration Using Titration Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved in a sample of known concentration. Concordant titres should be used when. Data collected during a titration allows chemists to determine a solution’s unknown concentration. The process of calculating concentration from titration data is described and illustrated. They can also be used to calculate the. How To Calculate Concentration Using Titration.
From www.youtube.com
Acid Base Titration Curves pH Calculations YouTube How To Calculate Concentration Using Titration In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly. Concordant titres should be used when. Titration calculations are used to find the concentration of unknown solutions. When the reaction between the analyte and titrant. They can also be used to calculate the ph after a given point during a titration. Titration is a method. How To Calculate Concentration Using Titration.
From www.youtube.com
Finding concentration of acid or base given amount needed to titrate How To Calculate Concentration Using Titration The process of calculating concentration from titration data is described and illustrated. The concentration of a species in solution can be determined by quantitative analysis. To calculate concentration, we need to know the amount of naoh and the volume of solution in which it is dissolved. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is. How To Calculate Concentration Using Titration.
From general.chemistrysteps.com
Strong AcidStrong Base Titrations Chemistry Steps How To Calculate Concentration Using Titration The concentration of a species in solution can be determined by quantitative analysis. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly. When the reaction between the analyte and titrant. To calculate concentration, we need to know the amount of naoh and the volume of solution in which it is dissolved. They can also. How To Calculate Concentration Using Titration.
From www.slideserve.com
PPT TITRATION PowerPoint Presentation, free download ID1459481 How To Calculate Concentration Using Titration Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved in a sample of known concentration. They can also be used to calculate the ph after a given point during a titration. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly. One such method is a titration, in which. How To Calculate Concentration Using Titration.
From www.studypool.com
SOLUTION Using titrations to calculate concentration Studypool How To Calculate Concentration Using Titration The concentration of a species in solution can be determined by quantitative analysis. Concordant titres should be used when. When the reaction between the analyte and titrant. Data collected during a titration allows chemists to determine a solution’s unknown concentration. They can also be used to calculate the ph after a given point during a titration. The process of calculating. How To Calculate Concentration Using Titration.
From www.wikihow.com
5 Easy Ways to Calculate the Concentration of a Solution How To Calculate Concentration Using Titration Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved in a sample of known concentration. To calculate concentration, we need to know the amount of naoh and the volume of solution in which it is dissolved. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly. They can also. How To Calculate Concentration Using Titration.
From www.youtube.com
Acid Base Titration Problems, Basic Introduction, Calculations How To Calculate Concentration Using Titration Titration calculations are used to find the concentration of unknown solutions. Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved in a sample of known concentration. The process of calculating concentration from titration data is described and illustrated. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly. They. How To Calculate Concentration Using Titration.
From www.youtube.com
The titration of 25 0 mL of an unknown concentration of H2SO4 solution How To Calculate Concentration Using Titration One such method is a titration, in which a measured volume of a solution of one substance, the titrant, is. Concordant titres should be used when. The concentration of a species in solution can be determined by quantitative analysis. The former quantity could be obtained via a stoichiometric ratio from the. To calculate concentration, we need to know the amount. How To Calculate Concentration Using Titration.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple How To Calculate Concentration Using Titration Data collected during a titration allows chemists to determine a solution’s unknown concentration. The former quantity could be obtained via a stoichiometric ratio from the. Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved in a sample of known concentration. Concordant titres should be used when. The process of calculating concentration from titration data. How To Calculate Concentration Using Titration.
From ceavsgvd.blob.core.windows.net
How To Calculate Acid Concentration From Titration at Susan Carrier blog How To Calculate Concentration Using Titration The former quantity could be obtained via a stoichiometric ratio from the. Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved in a sample of known concentration. Titration calculations are used to find the concentration of unknown solutions. They can also be used to calculate the ph after a given point during a titration.. How To Calculate Concentration Using Titration.
From www.youtube.com
An Introduction To Titration Calculations (GCSE Chemistry) YouTube How To Calculate Concentration Using Titration Concordant titres should be used when. To calculate concentration, we need to know the amount of naoh and the volume of solution in which it is dissolved. They can also be used to calculate the ph after a given point during a titration. The former quantity could be obtained via a stoichiometric ratio from the. The process of calculating concentration. How To Calculate Concentration Using Titration.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation How To Calculate Concentration Using Titration They can also be used to calculate the ph after a given point during a titration. Data collected during a titration allows chemists to determine a solution’s unknown concentration. The process of calculating concentration from titration data is described and illustrated. When the reaction between the analyte and titrant. Titration is a method to determine the unknown concentration of a. How To Calculate Concentration Using Titration.
From www.slideserve.com
PPT Calculating concentrations PowerPoint Presentation, free download How To Calculate Concentration Using Titration Data collected during a titration allows chemists to determine a solution’s unknown concentration. Titration calculations are used to find the concentration of unknown solutions. The former quantity could be obtained via a stoichiometric ratio from the. When the reaction between the analyte and titrant. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly. The. How To Calculate Concentration Using Titration.
From childhealthpolicy.vumc.org
🐈 Titration experiment results. How do you report a titration How To Calculate Concentration Using Titration The former quantity could be obtained via a stoichiometric ratio from the. To calculate concentration, we need to know the amount of naoh and the volume of solution in which it is dissolved. The process of calculating concentration from titration data is described and illustrated. Data collected during a titration allows chemists to determine a solution’s unknown concentration. Concordant titres. How To Calculate Concentration Using Titration.
From ceavsgvd.blob.core.windows.net
How To Calculate Acid Concentration From Titration at Susan Carrier blog How To Calculate Concentration Using Titration Data collected during a titration allows chemists to determine a solution’s unknown concentration. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly. They can also be used to calculate the ph after a given point during a titration. The process of calculating concentration from titration data is described and illustrated. The former quantity could. How To Calculate Concentration Using Titration.