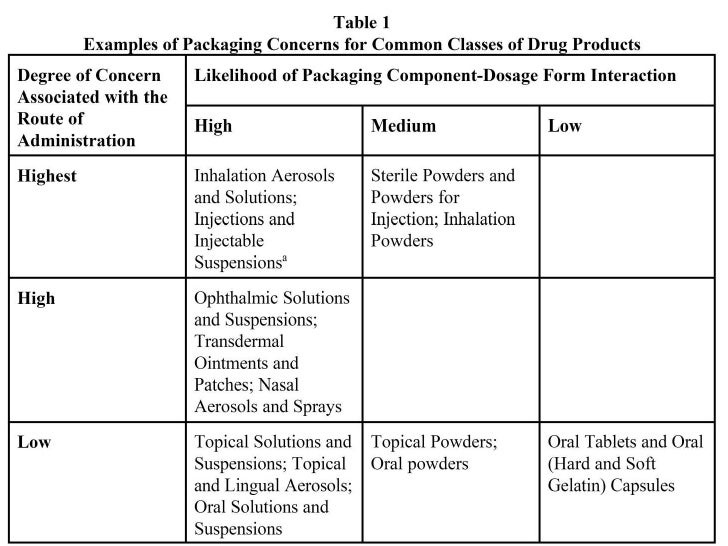
Container Closure Compatibility Study . Abstract container closure integrity (cci) testing, along with other engineering and administrative controls, must be incorporated into a holistic. A gap or breach in the container capable of permitting the passage of liquid or gas. The knowledge gained from the studies investigating the potential effect of drug substance properties on drug product. Container closure integrity (cci) testing, along with other engineering and administrative controls, must be incorporated. A possible interaction between product and container or. Stability recommendations for container closure systems of different types are described in the guideline for submitting documentation for the. Describe studies performed to demonstrate the integrity of the container and closure. Container closure integrity is considered an essential part of suitability, especially in the aspect of protection against microbial. Otherwise known as “leak path.”
from www.slideshare.net
A possible interaction between product and container or. Abstract container closure integrity (cci) testing, along with other engineering and administrative controls, must be incorporated into a holistic. Container closure integrity (cci) testing, along with other engineering and administrative controls, must be incorporated. Otherwise known as “leak path.” Stability recommendations for container closure systems of different types are described in the guideline for submitting documentation for the. A gap or breach in the container capable of permitting the passage of liquid or gas. Describe studies performed to demonstrate the integrity of the container and closure. The knowledge gained from the studies investigating the potential effect of drug substance properties on drug product. Container closure integrity is considered an essential part of suitability, especially in the aspect of protection against microbial.
FDA container closure system & drug stability saurav anand 23 iip
Container Closure Compatibility Study Describe studies performed to demonstrate the integrity of the container and closure. Container closure integrity is considered an essential part of suitability, especially in the aspect of protection against microbial. Abstract container closure integrity (cci) testing, along with other engineering and administrative controls, must be incorporated into a holistic. Stability recommendations for container closure systems of different types are described in the guideline for submitting documentation for the. Describe studies performed to demonstrate the integrity of the container and closure. Otherwise known as “leak path.” The knowledge gained from the studies investigating the potential effect of drug substance properties on drug product. A possible interaction between product and container or. Container closure integrity (cci) testing, along with other engineering and administrative controls, must be incorporated. A gap or breach in the container capable of permitting the passage of liquid or gas.
From dokumen.tips
(PDF) Container Closure Integrity Evaluation for Sterile Product Container Closure Compatibility Study Container closure integrity is considered an essential part of suitability, especially in the aspect of protection against microbial. A possible interaction between product and container or. Stability recommendations for container closure systems of different types are described in the guideline for submitting documentation for the. A gap or breach in the container capable of permitting the passage of liquid or. Container Closure Compatibility Study.
From www.slideshare.net
Ich guidelines for stability studies 1 Container Closure Compatibility Study Otherwise known as “leak path.” The knowledge gained from the studies investigating the potential effect of drug substance properties on drug product. Container closure integrity (cci) testing, along with other engineering and administrative controls, must be incorporated. Abstract container closure integrity (cci) testing, along with other engineering and administrative controls, must be incorporated into a holistic. A gap or breach. Container Closure Compatibility Study.
From labbyag.es
Injectable Drug Compatibility Chart Iv Drug Compatibility Chart Iv Container Closure Compatibility Study A gap or breach in the container capable of permitting the passage of liquid or gas. Container closure integrity (cci) testing, along with other engineering and administrative controls, must be incorporated. Container closure integrity is considered an essential part of suitability, especially in the aspect of protection against microbial. Describe studies performed to demonstrate the integrity of the container and. Container Closure Compatibility Study.
From www.gaplast.de
Container Closure Systems GAPLAST Container Closure Compatibility Study Otherwise known as “leak path.” Abstract container closure integrity (cci) testing, along with other engineering and administrative controls, must be incorporated into a holistic. The knowledge gained from the studies investigating the potential effect of drug substance properties on drug product. A gap or breach in the container capable of permitting the passage of liquid or gas. Container closure integrity. Container Closure Compatibility Study.
From www.pinterest.com
Container Closure Integrity Testing (CCIT) An Ideal Solution for the Container Closure Compatibility Study A gap or breach in the container capable of permitting the passage of liquid or gas. The knowledge gained from the studies investigating the potential effect of drug substance properties on drug product. A possible interaction between product and container or. Container closure integrity (cci) testing, along with other engineering and administrative controls, must be incorporated. Describe studies performed to. Container Closure Compatibility Study.
From insideofpharma.blogspot.com
Pharma Knowledge Container Closure Integrity Test Container Closure Compatibility Study Describe studies performed to demonstrate the integrity of the container and closure. Otherwise known as “leak path.” A possible interaction between product and container or. A gap or breach in the container capable of permitting the passage of liquid or gas. The knowledge gained from the studies investigating the potential effect of drug substance properties on drug product. Container closure. Container Closure Compatibility Study.
From pdfslide.net
Parenteral Container/Closure .Selection and Utilization of Parenteral Container Closure Compatibility Study Container closure integrity (cci) testing, along with other engineering and administrative controls, must be incorporated. Container closure integrity is considered an essential part of suitability, especially in the aspect of protection against microbial. Otherwise known as “leak path.” Abstract container closure integrity (cci) testing, along with other engineering and administrative controls, must be incorporated into a holistic. Describe studies performed. Container Closure Compatibility Study.
From app.livestorm.co
Container Closure Integrity Testing Deterministic Methods Easyfairs Container Closure Compatibility Study Abstract container closure integrity (cci) testing, along with other engineering and administrative controls, must be incorporated into a holistic. Describe studies performed to demonstrate the integrity of the container and closure. Container closure integrity is considered an essential part of suitability, especially in the aspect of protection against microbial. Stability recommendations for container closure systems of different types are described. Container Closure Compatibility Study.
From www.linkedin.com
Container Closure Integrity Testing Solutions Offered by PTI for Biologics Container Closure Compatibility Study Container closure integrity (cci) testing, along with other engineering and administrative controls, must be incorporated. The knowledge gained from the studies investigating the potential effect of drug substance properties on drug product. Otherwise known as “leak path.” Describe studies performed to demonstrate the integrity of the container and closure. Container closure integrity is considered an essential part of suitability, especially. Container Closure Compatibility Study.
From www.pti-ccit.com
Container Closure Integrity Testing using VeriPac 355 Technology Container Closure Compatibility Study A gap or breach in the container capable of permitting the passage of liquid or gas. Stability recommendations for container closure systems of different types are described in the guideline for submitting documentation for the. Container closure integrity (cci) testing, along with other engineering and administrative controls, must be incorporated. Otherwise known as “leak path.” The knowledge gained from the. Container Closure Compatibility Study.
From www.slideserve.com
PPT Container closure system PowerPoint Presentation, free download Container Closure Compatibility Study A possible interaction between product and container or. Describe studies performed to demonstrate the integrity of the container and closure. Container closure integrity (cci) testing, along with other engineering and administrative controls, must be incorporated. Otherwise known as “leak path.” Container closure integrity is considered an essential part of suitability, especially in the aspect of protection against microbial. The knowledge. Container Closure Compatibility Study.
From www.slideteam.net
Container Closure System Description And Suitability Pharmaceutical Container Closure Compatibility Study A gap or breach in the container capable of permitting the passage of liquid or gas. Otherwise known as “leak path.” A possible interaction between product and container or. Container closure integrity (cci) testing, along with other engineering and administrative controls, must be incorporated. The knowledge gained from the studies investigating the potential effect of drug substance properties on drug. Container Closure Compatibility Study.
From www.a3p.org
Container Closure Integrity Testing of Sterile Injectable Products Container Closure Compatibility Study Container closure integrity (cci) testing, along with other engineering and administrative controls, must be incorporated. Stability recommendations for container closure systems of different types are described in the guideline for submitting documentation for the. A possible interaction between product and container or. Describe studies performed to demonstrate the integrity of the container and closure. The knowledge gained from the studies. Container Closure Compatibility Study.
From www.researchgate.net
(PDF) ContainerContent Compatibility Studies A Pharmaceutical Team's Container Closure Compatibility Study The knowledge gained from the studies investigating the potential effect of drug substance properties on drug product. A possible interaction between product and container or. Abstract container closure integrity (cci) testing, along with other engineering and administrative controls, must be incorporated into a holistic. Describe studies performed to demonstrate the integrity of the container and closure. Container closure integrity is. Container Closure Compatibility Study.
From www.pda.org
Awareness Critical for Container Closure Components PDA Container Closure Compatibility Study Container closure integrity is considered an essential part of suitability, especially in the aspect of protection against microbial. Stability recommendations for container closure systems of different types are described in the guideline for submitting documentation for the. A gap or breach in the container capable of permitting the passage of liquid or gas. The knowledge gained from the studies investigating. Container Closure Compatibility Study.
From www.slideserve.com
PPT FPP assessment approaches and considerations PowerPoint Container Closure Compatibility Study A gap or breach in the container capable of permitting the passage of liquid or gas. Stability recommendations for container closure systems of different types are described in the guideline for submitting documentation for the. A possible interaction between product and container or. The knowledge gained from the studies investigating the potential effect of drug substance properties on drug product.. Container Closure Compatibility Study.
From cargorestraintsystems.com.au
IBC in Containers Secured with Cordstrap Cross Lashing and Harness Container Closure Compatibility Study Container closure integrity (cci) testing, along with other engineering and administrative controls, must be incorporated. Stability recommendations for container closure systems of different types are described in the guideline for submitting documentation for the. Abstract container closure integrity (cci) testing, along with other engineering and administrative controls, must be incorporated into a holistic. Otherwise known as “leak path.” A possible. Container Closure Compatibility Study.
From www.slideserve.com
PPT Container closure system PowerPoint Presentation, free download Container Closure Compatibility Study Otherwise known as “leak path.” Stability recommendations for container closure systems of different types are described in the guideline for submitting documentation for the. The knowledge gained from the studies investigating the potential effect of drug substance properties on drug product. A possible interaction between product and container or. Abstract container closure integrity (cci) testing, along with other engineering and. Container Closure Compatibility Study.
From slideplayer.in.th
Manufacturing of Drug Product ppt ดาวน์โหลด Container Closure Compatibility Study Container closure integrity (cci) testing, along with other engineering and administrative controls, must be incorporated. Container closure integrity is considered an essential part of suitability, especially in the aspect of protection against microbial. Describe studies performed to demonstrate the integrity of the container and closure. Abstract container closure integrity (cci) testing, along with other engineering and administrative controls, must be. Container Closure Compatibility Study.
From www.cphi-online.com
ZebraSci Inc. Container Closure Integrity Testing Deterministic Container Closure Compatibility Study Container closure integrity is considered an essential part of suitability, especially in the aspect of protection against microbial. A gap or breach in the container capable of permitting the passage of liquid or gas. A possible interaction between product and container or. Container closure integrity (cci) testing, along with other engineering and administrative controls, must be incorporated. The knowledge gained. Container Closure Compatibility Study.
From studylib.net
container closure instructions Heritage Container Closure Compatibility Study The knowledge gained from the studies investigating the potential effect of drug substance properties on drug product. Abstract container closure integrity (cci) testing, along with other engineering and administrative controls, must be incorporated into a holistic. Describe studies performed to demonstrate the integrity of the container and closure. A possible interaction between product and container or. Otherwise known as “leak. Container Closure Compatibility Study.
From www.silgancls.com
plastic closure terminology Silgan Closures Container Closure Compatibility Study Stability recommendations for container closure systems of different types are described in the guideline for submitting documentation for the. Otherwise known as “leak path.” A gap or breach in the container capable of permitting the passage of liquid or gas. Abstract container closure integrity (cci) testing, along with other engineering and administrative controls, must be incorporated into a holistic. Container. Container Closure Compatibility Study.
From www.slideserve.com
PPT Compatibility Testing (cross matching) PowerPoint Presentation Container Closure Compatibility Study Otherwise known as “leak path.” Stability recommendations for container closure systems of different types are described in the guideline for submitting documentation for the. Describe studies performed to demonstrate the integrity of the container and closure. A possible interaction between product and container or. Container closure integrity (cci) testing, along with other engineering and administrative controls, must be incorporated. Container. Container Closure Compatibility Study.
From www.pda.org
Awareness Critical for Container Closure Components PDA Container Closure Compatibility Study Describe studies performed to demonstrate the integrity of the container and closure. Otherwise known as “leak path.” A gap or breach in the container capable of permitting the passage of liquid or gas. Container closure integrity (cci) testing, along with other engineering and administrative controls, must be incorporated. Stability recommendations for container closure systems of different types are described in. Container Closure Compatibility Study.
From lighthouseinstruments.com
Lighthouse Container Closure Integrity inar Series Lighthouse Container Closure Compatibility Study A possible interaction between product and container or. Container closure integrity is considered an essential part of suitability, especially in the aspect of protection against microbial. A gap or breach in the container capable of permitting the passage of liquid or gas. Otherwise known as “leak path.” Stability recommendations for container closure systems of different types are described in the. Container Closure Compatibility Study.
From www.slideshare.net
FDA container closure system & drug stability saurav anand 23 iip Container Closure Compatibility Study Describe studies performed to demonstrate the integrity of the container and closure. A possible interaction between product and container or. Stability recommendations for container closure systems of different types are described in the guideline for submitting documentation for the. Container closure integrity (cci) testing, along with other engineering and administrative controls, must be incorporated. Abstract container closure integrity (cci) testing,. Container Closure Compatibility Study.
From www.pti-ccit.com
Container Closure Integrity Techniques for Pharmaceutical Package Integrity Container Closure Compatibility Study The knowledge gained from the studies investigating the potential effect of drug substance properties on drug product. A gap or breach in the container capable of permitting the passage of liquid or gas. Otherwise known as “leak path.” Container closure integrity (cci) testing, along with other engineering and administrative controls, must be incorporated. Stability recommendations for container closure systems of. Container Closure Compatibility Study.
From www.scribd.com
Developing a Strategic Approach to Container Closure Integrity Testing Container Closure Compatibility Study Describe studies performed to demonstrate the integrity of the container and closure. Otherwise known as “leak path.” Abstract container closure integrity (cci) testing, along with other engineering and administrative controls, must be incorporated into a holistic. Container closure integrity is considered an essential part of suitability, especially in the aspect of protection against microbial. Container closure integrity (cci) testing, along. Container Closure Compatibility Study.
From www.syntegon.com
Container Closure Integrity » Syntegon Container Closure Compatibility Study Abstract container closure integrity (cci) testing, along with other engineering and administrative controls, must be incorporated into a holistic. Describe studies performed to demonstrate the integrity of the container and closure. Container closure integrity (cci) testing, along with other engineering and administrative controls, must be incorporated. Container closure integrity is considered an essential part of suitability, especially in the aspect. Container Closure Compatibility Study.
From www.pti-ccit.com
Evaluating Container Closure Integrity of HighRisk Pharmaceuticals Container Closure Compatibility Study Stability recommendations for container closure systems of different types are described in the guideline for submitting documentation for the. Container closure integrity (cci) testing, along with other engineering and administrative controls, must be incorporated. A gap or breach in the container capable of permitting the passage of liquid or gas. The knowledge gained from the studies investigating the potential effect. Container Closure Compatibility Study.
From www.cellandgene.com
Container Closure Integrity Testing Strategies For Gene Therapies Container Closure Compatibility Study Container closure integrity is considered an essential part of suitability, especially in the aspect of protection against microbial. Abstract container closure integrity (cci) testing, along with other engineering and administrative controls, must be incorporated into a holistic. Otherwise known as “leak path.” Describe studies performed to demonstrate the integrity of the container and closure. The knowledge gained from the studies. Container Closure Compatibility Study.
From www.pda.org
Awareness Critical for Container Closure Components PDA Container Closure Compatibility Study Container closure integrity (cci) testing, along with other engineering and administrative controls, must be incorporated. Describe studies performed to demonstrate the integrity of the container and closure. Abstract container closure integrity (cci) testing, along with other engineering and administrative controls, must be incorporated into a holistic. Container closure integrity is considered an essential part of suitability, especially in the aspect. Container Closure Compatibility Study.
From mungfali.com
Container Closure Integrity Test Container Closure Compatibility Study Stability recommendations for container closure systems of different types are described in the guideline for submitting documentation for the. Describe studies performed to demonstrate the integrity of the container and closure. Container closure integrity is considered an essential part of suitability, especially in the aspect of protection against microbial. Otherwise known as “leak path.” A gap or breach in the. Container Closure Compatibility Study.
From www.scribd.com
Container Closure System PDF Applied And Interdisciplinary Physics Container Closure Compatibility Study Otherwise known as “leak path.” A possible interaction between product and container or. Describe studies performed to demonstrate the integrity of the container and closure. The knowledge gained from the studies investigating the potential effect of drug substance properties on drug product. Stability recommendations for container closure systems of different types are described in the guideline for submitting documentation for. Container Closure Compatibility Study.
From www.heliumleak.com
Testing Container Closure Integrity at Low Temperature Container Closure Compatibility Study The knowledge gained from the studies investigating the potential effect of drug substance properties on drug product. Abstract container closure integrity (cci) testing, along with other engineering and administrative controls, must be incorporated into a holistic. Stability recommendations for container closure systems of different types are described in the guideline for submitting documentation for the. A gap or breach in. Container Closure Compatibility Study.