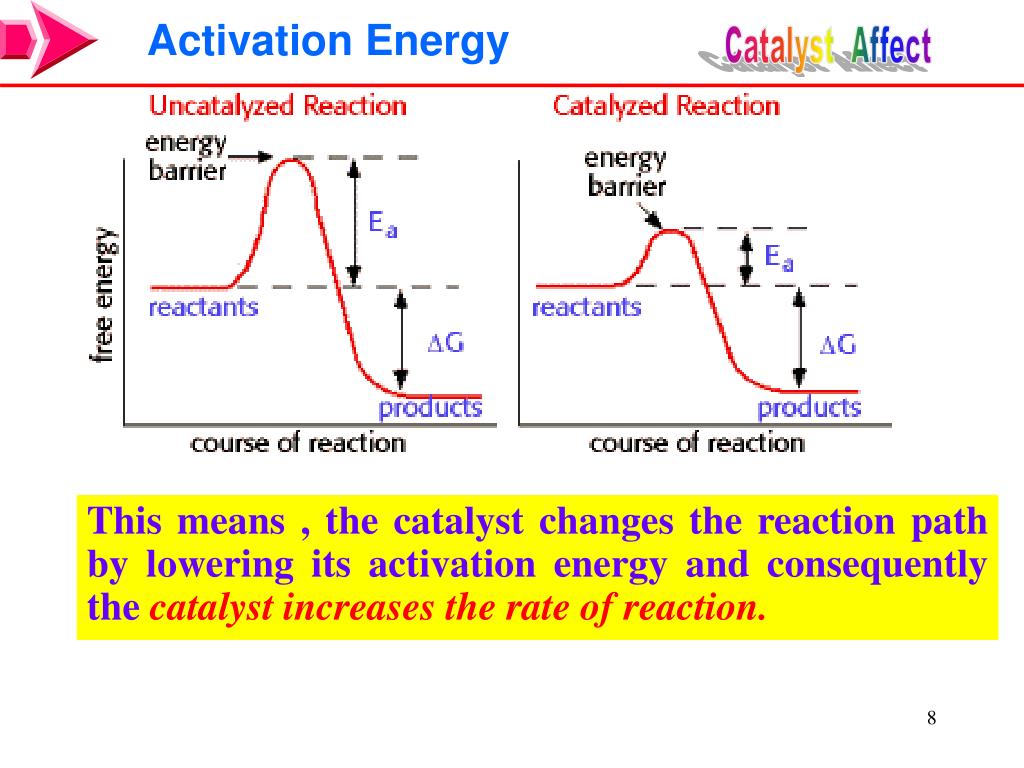
Catalysts With Reactions . There are two main ways that catalysts work. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more favorable thermodynamically. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. They increase the rate of reaction, are not consumed by the reaction and are only needed in very small amounts. What are catalysts, and how do they work in terms altering the parameters of a reaction? Catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. This process is called catalysis. Catalysts play an important part in many chemical processes. Enzymes are naturally occurring catalysts responsible for many. A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. Catalysts reduce the activation energy of reactions and enhance the rate of specific reactions. Therefore they are crucially important in chemical industry, exhaust gas treatment and. A catalyst is a chemical substance.
from www.slideserve.com
Catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. Enzymes are naturally occurring catalysts responsible for many. Catalysts reduce the activation energy of reactions and enhance the rate of specific reactions. They increase the rate of reaction, are not consumed by the reaction and are only needed in very small amounts. A catalyst is a chemical substance. There are two main ways that catalysts work. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Therefore they are crucially important in chemical industry, exhaust gas treatment and. This process is called catalysis. What are catalysts, and how do they work in terms altering the parameters of a reaction?
PPT Mechanisms of Catalytic Reactions and Characterization of
Catalysts With Reactions What are catalysts, and how do they work in terms altering the parameters of a reaction? A catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more favorable thermodynamically. This process is called catalysis. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysts reduce the activation energy of reactions and enhance the rate of specific reactions. A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. Catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. A catalyst is a chemical substance. Catalysts play an important part in many chemical processes. Enzymes are naturally occurring catalysts responsible for many. What are catalysts, and how do they work in terms altering the parameters of a reaction? There are two main ways that catalysts work. They increase the rate of reaction, are not consumed by the reaction and are only needed in very small amounts. Therefore they are crucially important in chemical industry, exhaust gas treatment and.
From nonsibihighschool.org
Section 184 Catalysis Catalysts With Reactions What are catalysts, and how do they work in terms altering the parameters of a reaction? They increase the rate of reaction, are not consumed by the reaction and are only needed in very small amounts. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to. Catalysts With Reactions.
From www.pinterest.com
Catalyst speeds up a chemical reaction by lowering the activation Catalysts With Reactions They increase the rate of reaction, are not consumed by the reaction and are only needed in very small amounts. What are catalysts, and how do they work in terms altering the parameters of a reaction? A catalyst is a chemical substance. Catalysts reduce the activation energy of reactions and enhance the rate of specific reactions. Catalysts play an important. Catalysts With Reactions.
From wiringfixunripping.z21.web.core.windows.net
Reaction Energy Diagram With Catalyst Catalysts With Reactions They increase the rate of reaction, are not consumed by the reaction and are only needed in very small amounts. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. Catalysts play an important part in many chemical processes. Therefore they are crucially important in. Catalysts With Reactions.
From www.youtube.com
Catalysts AP Chemistry Khan Academy YouTube Catalysts With Reactions Catalysts reduce the activation energy of reactions and enhance the rate of specific reactions. Catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. A catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more favorable thermodynamically. There are two main ways. Catalysts With Reactions.
From ar.inspiredpencil.com
Catalyst Reaction Diagram Catalysts With Reactions They increase the rate of reaction, are not consumed by the reaction and are only needed in very small amounts. Therefore they are crucially important in chemical industry, exhaust gas treatment and. Catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. What are catalysts, and how do they work in terms. Catalysts With Reactions.
From www.slideserve.com
PPT CATALYSIS AND CATALYTIC REACTION MECHANISM PART 1 PowerPoint Catalysts With Reactions A catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more favorable thermodynamically. They increase the rate of reaction, are not consumed by the reaction and are only needed in very small amounts. Therefore they are crucially important in chemical industry, exhaust gas treatment and. A catalyst is a chemical. Catalysts With Reactions.
From www.savemyexams.co.uk
Catalysts (1.7.6) AQA A Level Chemistry Revision Notes 2017 Save My Catalysts With Reactions Catalysts play an important part in many chemical processes. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysts reduce the activation energy of reactions and enhance the rate of specific reactions. Therefore they are crucially important in chemical industry, exhaust gas treatment and. A catalyst is a chemical substance. They increase the. Catalysts With Reactions.
From kenya-khurst.blogspot.com
Catalysts Lower the Activation Energy of a Reaction by Catalysts With Reactions Catalysts play an important part in many chemical processes. There are two main ways that catalysts work. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. What are catalysts, and how do they work in terms altering the parameters of a reaction? They increase the rate of reaction, are not consumed by the. Catalysts With Reactions.
From www.slideserve.com
PPT Mechanisms of Catalytic Reactions and Characterization of Catalysts With Reactions Catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. Enzymes are naturally occurring catalysts responsible for many. Catalysts reduce the activation energy of reactions and enhance the rate of specific reactions. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required. Catalysts With Reactions.
From www.dreamstime.com
Catalyst Surface with Catalytic Reaction Stock Vector Illustration of Catalysts With Reactions Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. Catalysts. Catalysts With Reactions.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii Catalysts With Reactions Catalysts play an important part in many chemical processes. Therefore they are crucially important in chemical industry, exhaust gas treatment and. Catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. This process is. Catalysts With Reactions.
From www.researchgate.net
Scheme 4.5 the three reactions catalyzed by the solid acid catalyst Catalysts With Reactions A catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more favorable thermodynamically. What are catalysts, and how do they work in terms altering the parameters of a reaction? Enzymes are naturally occurring catalysts responsible for many. A catalyst is a chemical substance. Catalysts play an important part in many. Catalysts With Reactions.
From derekcarrsavvy-chemist.blogspot.com
savvychemist GCSE OCR Gateway Chemistry C5.2 fi Catalysis and catalysts Catalysts With Reactions What are catalysts, and how do they work in terms altering the parameters of a reaction? A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. This process is called catalysis. They increase the rate of reaction, are not consumed by the reaction and are. Catalysts With Reactions.
From www.slideserve.com
PPT Chapter 11 Chemical Reactions PowerPoint Presentation, free Catalysts With Reactions A catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more favorable thermodynamically. Catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. They increase the rate of reaction, are not consumed by the reaction and are only needed in very small. Catalysts With Reactions.
From www.youtube.com
A Catalyst and the Rate of Reaction YouTube Catalysts With Reactions Therefore they are crucially important in chemical industry, exhaust gas treatment and. There are two main ways that catalysts work. A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. Catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. A catalyst binds to. Catalysts With Reactions.
From www.youtube.com
Identifying catalysts in a reaction YouTube Catalysts With Reactions Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. This process is called catalysis. Enzymes are naturally occurring catalysts responsible for many. Therefore they are crucially important in chemical industry, exhaust gas treatment and. Catalysts reduce the activation energy of reactions and enhance the rate of specific reactions. There are two main ways. Catalysts With Reactions.
From ar.inspiredpencil.com
Endothermic Reaction With Catalyst Catalysts With Reactions A catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more favorable thermodynamically. Catalysts reduce the activation energy of reactions and enhance the rate of specific reactions. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction. Catalysts With Reactions.
From www.slideshare.net
Catalyst Catalysts With Reactions A catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more favorable thermodynamically. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. Therefore they are crucially important in chemical industry, exhaust gas treatment and.. Catalysts With Reactions.
From www.alamy.com
Chemical Reactions of catalyst and product Stock Vector Image & Art Alamy Catalysts With Reactions A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. This process is called catalysis. What are catalysts, and how do they work in terms altering the parameters of a reaction? Catalysts reduce the activation energy of reactions and enhance the rate of specific reactions.. Catalysts With Reactions.
From large.stanford.edu
Catalysts in 21st Century Energy Catalysts With Reactions Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. What are catalysts, and how do they work in terms altering the parameters of a reaction? A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. This process is. Catalysts With Reactions.
From schoolbag.info
A catalyst speeds up a reaction by providing the reactants with an Catalysts With Reactions A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. They increase the rate of reaction, are not consumed by the reaction and are only needed in very small. Catalysts With Reactions.
From www.mometrix.com
What is a Catalyst? Chemistry Review (Video) Catalysts With Reactions A catalyst is a chemical substance. This process is called catalysis. What are catalysts, and how do they work in terms altering the parameters of a reaction? A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. Catalyst, in chemistry, any substance that increases the. Catalysts With Reactions.
From wou.edu
Chapter 7 Catalytic Mechanisms of Enzymes Chemistry Catalysts With Reactions What are catalysts, and how do they work in terms altering the parameters of a reaction? They increase the rate of reaction, are not consumed by the reaction and are only needed in very small amounts. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to. Catalysts With Reactions.
From www.meritnation.com
Illustrate graphically the effect of a catalyst on rate of a reaction Catalysts With Reactions What are catalysts, and how do they work in terms altering the parameters of a reaction? A catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more favorable thermodynamically. There are two main ways that catalysts work. Catalysis is the process that alters the rate of a chemical reaction under. Catalysts With Reactions.
From circuitdbplastered.z13.web.core.windows.net
Reaction Energy Diagram With Catalyst Catalysts With Reactions There are two main ways that catalysts work. This process is called catalysis. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. What are catalysts, and how do they work in terms altering the parameters of a reaction? A catalyst binds to a reactant. Catalysts With Reactions.
From www.ck12.org
Catalysts Example 1 ( Video ) Chemistry CK12 Foundation Catalysts With Reactions Therefore they are crucially important in chemical industry, exhaust gas treatment and. A catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more favorable thermodynamically. Catalysts play an important part in many chemical processes. What are catalysts, and how do they work in terms altering the parameters of a reaction?. Catalysts With Reactions.
From www.dreamstime.com
Enzyme As Catalyst in Chemical Reactions Stock Vector Illustration of Catalysts With Reactions What are catalysts, and how do they work in terms altering the parameters of a reaction? Catalysts play an important part in many chemical processes. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. Catalysis is the process that alters the rate of a. Catalysts With Reactions.
From www.waca.msf.org
Catalyst, Catalyst Catalysts With Reactions Therefore they are crucially important in chemical industry, exhaust gas treatment and. A catalyst is a chemical substance. Catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is not consumed by the reaction. Catalysts With Reactions.
From ar.inspiredpencil.com
Exothermic Reaction With Catalyst Catalysts With Reactions Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. They increase the rate of reaction, are not consumed by the reaction and are only needed in very small amounts. Enzymes are naturally occurring catalysts responsible for many. A catalyst binds to a reactant and it increases the number of collision between the reactant. Catalysts With Reactions.
From www.researchgate.net
Reaction coordinate diagram showing the working principle of a catalyst Catalysts With Reactions Therefore they are crucially important in chemical industry, exhaust gas treatment and. A catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more favorable thermodynamically. A catalyst is a chemical substance. What are catalysts, and how do they work in terms altering the parameters of a reaction? A catalyst is. Catalysts With Reactions.
From www.researchgate.net
Effect of catalyst on energy diagram profile. Download Scientific Diagram Catalysts With Reactions Catalysts reduce the activation energy of reactions and enhance the rate of specific reactions. Catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. Catalysts play an important part in many chemical processes. What are catalysts, and how do they work in terms altering the parameters of a reaction? There are two. Catalysts With Reactions.
From 2012books.lardbucket.org
Catalysis Catalysts With Reactions What are catalysts, and how do they work in terms altering the parameters of a reaction? Catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. A catalyst is a chemical substance. They increase the rate of reaction, are not consumed by the reaction and are only needed in very small amounts.. Catalysts With Reactions.
From www.researchgate.net
Catalytic processes on a solid catalyst. Download Scientific Diagram Catalysts With Reactions What are catalysts, and how do they work in terms altering the parameters of a reaction? Catalysts play an important part in many chemical processes. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Therefore they are crucially important in chemical industry, exhaust gas treatment and. A catalyst is a chemical substance that. Catalysts With Reactions.
From www.slideserve.com
PPT CATALYSIS AND CATALYTIC REACTION MECHANISM PART 1 PowerPoint Catalysts With Reactions There are two main ways that catalysts work. Catalysts reduce the activation energy of reactions and enhance the rate of specific reactions. This process is called catalysis. They increase the rate of reaction, are not consumed by the reaction and are only needed in very small amounts. A catalyst is a chemical substance that affects the rate of a chemical. Catalysts With Reactions.
From www.researchgate.net
1 Schematic illustration of a catalytic process showing "A" and "B Catalysts With Reactions A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. They increase the rate of reaction, are not consumed by the reaction and are only needed in very small amounts. This process is called catalysis. A catalyst binds to a reactant and it increases the. Catalysts With Reactions.