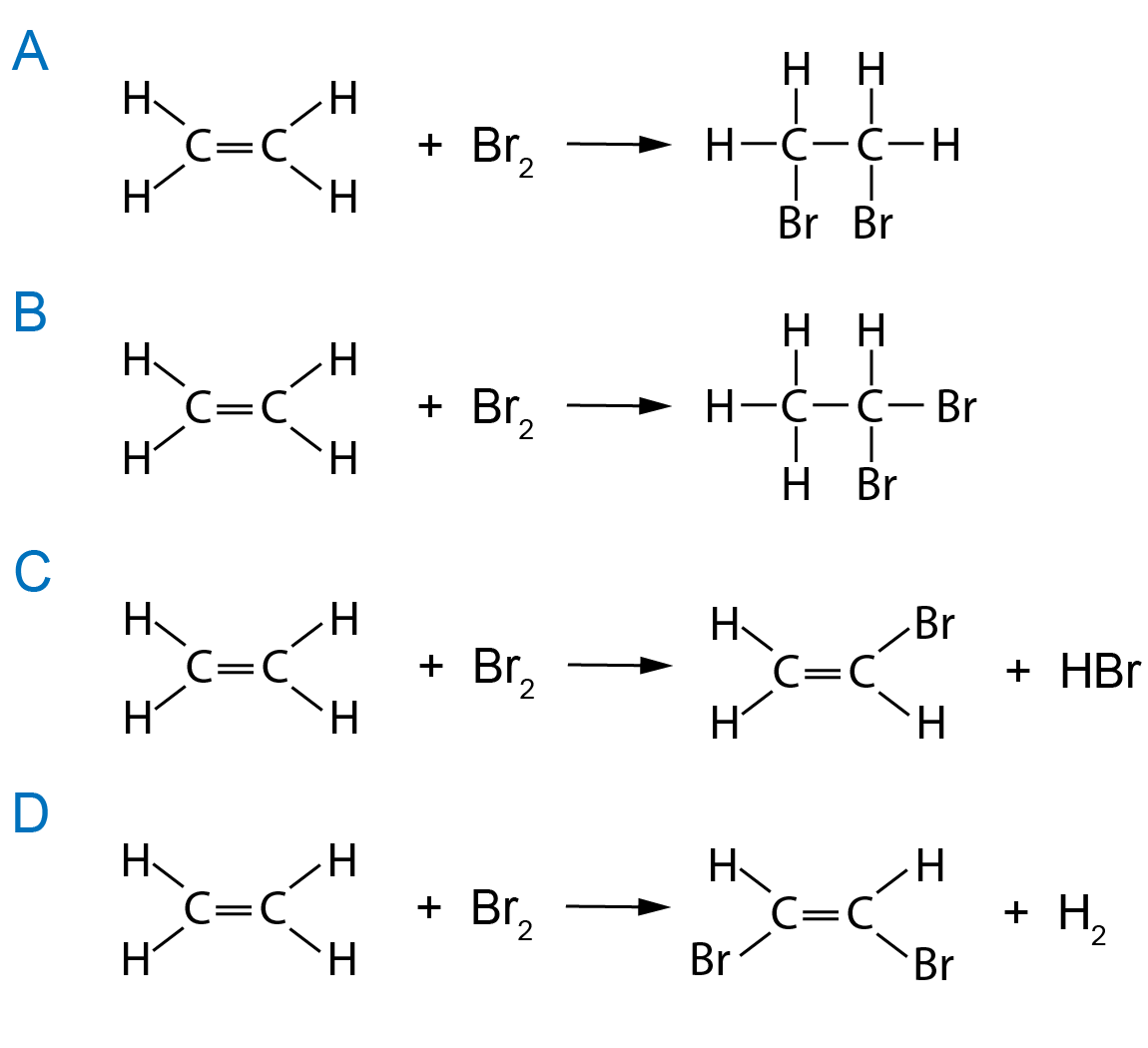Bromine In Water Reaction . When bromine is added to the sample, if the reddish color disappear, that means the sample does contain an alkene. All alkanes are saturated and alkenes are unsaturated; The presence of the c=c double bond allows alkenes to react in ways that alkanes cannot; Bromine water is a yellow mixture solution with high oxidizing properties, prepared by dissolving diatomic bromine (br 2) in water (h 2 o). This allows us to tell alkenes apart from alkanes using a simple chemical test called the bromine water test The addition reaction occurs to get reddish bromine consumed and colorless product formed, so color fades off. Alkanes and alkenes have different molecular structures; Bromine water solution can be prepared in the chemistry lab by direct mixing of fumes of bromine and water. When bromine is added to the sample, if the reddish color disappears, it means the sample contains an alkene. The reaction with bromine happens at room temperature. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. If you have a gaseous alkene like ethene, you can bubble it through either pure. The products for addition of halogen to alkenes seems straightforward, with each halogen added to each double bond carbon. The addition reaction occurs to get reddish bromine consumed and a.
from mungfali.com
The addition reaction occurs to get reddish bromine consumed and a. The presence of the c=c double bond allows alkenes to react in ways that alkanes cannot; The addition reaction occurs to get reddish bromine consumed and colorless product formed, so color fades off. The reaction with bromine happens at room temperature. All alkanes are saturated and alkenes are unsaturated; If you have a gaseous alkene like ethene, you can bubble it through either pure. This allows us to tell alkenes apart from alkanes using a simple chemical test called the bromine water test When bromine is added to the sample, if the reddish color disappear, that means the sample does contain an alkene. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. Bromine water solution can be prepared in the chemistry lab by direct mixing of fumes of bromine and water.
Ethene And Water Reaction
Bromine In Water Reaction The addition reaction occurs to get reddish bromine consumed and a. Alkanes and alkenes have different molecular structures; Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. Bromine water solution can be prepared in the chemistry lab by direct mixing of fumes of bromine and water. The presence of the c=c double bond allows alkenes to react in ways that alkanes cannot; The products for addition of halogen to alkenes seems straightforward, with each halogen added to each double bond carbon. The reaction with bromine happens at room temperature. When bromine is added to the sample, if the reddish color disappears, it means the sample contains an alkene. The addition reaction occurs to get reddish bromine consumed and colorless product formed, so color fades off. If you have a gaseous alkene like ethene, you can bubble it through either pure. Bromine water is a yellow mixture solution with high oxidizing properties, prepared by dissolving diatomic bromine (br 2) in water (h 2 o). The addition reaction occurs to get reddish bromine consumed and a. All alkanes are saturated and alkenes are unsaturated; This allows us to tell alkenes apart from alkanes using a simple chemical test called the bromine water test When bromine is added to the sample, if the reddish color disappear, that means the sample does contain an alkene.
From www.youtube.com
Identifying Alkenes with Bromine Water YouTube Bromine In Water Reaction Bromine water is a yellow mixture solution with high oxidizing properties, prepared by dissolving diatomic bromine (br 2) in water (h 2 o). Alkanes and alkenes have different molecular structures; The addition reaction occurs to get reddish bromine consumed and a. When bromine is added to the sample, if the reddish color disappear, that means the sample does contain an. Bromine In Water Reaction.
From joelgordon.photoshelter.com
Reaction of bromine with an unsaturated hydrocarbon Joel Gordon Bromine In Water Reaction If you have a gaseous alkene like ethene, you can bubble it through either pure. Bromine water is a yellow mixture solution with high oxidizing properties, prepared by dissolving diatomic bromine (br 2) in water (h 2 o). All alkanes are saturated and alkenes are unsaturated; When bromine is added to the sample, if the reddish color disappears, it means. Bromine In Water Reaction.
From www.sciencephoto.com
Bromine test for alkene Stock Image A500/0438 Science Photo Library Bromine In Water Reaction The reaction with bromine happens at room temperature. If you have a gaseous alkene like ethene, you can bubble it through either pure. When bromine is added to the sample, if the reddish color disappear, that means the sample does contain an alkene. This allows us to tell alkenes apart from alkanes using a simple chemical test called the bromine. Bromine In Water Reaction.
From www.youtube.com
Bromine Water Test For Aniline Reaction Of Aniline With Bromine Water Bromine In Water Reaction Bromine water is a yellow mixture solution with high oxidizing properties, prepared by dissolving diatomic bromine (br 2) in water (h 2 o). Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. The addition reaction occurs to get reddish bromine consumed and a. When bromine is added to. Bromine In Water Reaction.
From www.youtube.com
Bromine Water Test Explained// HSC Chemistry YouTube Bromine In Water Reaction Bromine water is a yellow mixture solution with high oxidizing properties, prepared by dissolving diatomic bromine (br 2) in water (h 2 o). If you have a gaseous alkene like ethene, you can bubble it through either pure. When bromine is added to the sample, if the reddish color disappears, it means the sample contains an alkene. Alkenes react in. Bromine In Water Reaction.
From byjus.com
How do you test for an alkene? Bromine In Water Reaction Alkanes and alkenes have different molecular structures; When bromine is added to the sample, if the reddish color disappears, it means the sample contains an alkene. The presence of the c=c double bond allows alkenes to react in ways that alkanes cannot; Bromine water solution can be prepared in the chemistry lab by direct mixing of fumes of bromine and. Bromine In Water Reaction.
From fineartamerica.com
Alkene Decolourisation Of Bromine Water Photograph by Andrew Lambert Bromine In Water Reaction All alkanes are saturated and alkenes are unsaturated; Bromine water is a yellow mixture solution with high oxidizing properties, prepared by dissolving diatomic bromine (br 2) in water (h 2 o). Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. Bromine water solution can be prepared in the. Bromine In Water Reaction.
From www.nagwa.com
Question Video Identifying the Product of the Reaction of Ethyne Gas Bromine In Water Reaction The products for addition of halogen to alkenes seems straightforward, with each halogen added to each double bond carbon. Alkanes and alkenes have different molecular structures; The addition reaction occurs to get reddish bromine consumed and a. The presence of the c=c double bond allows alkenes to react in ways that alkanes cannot; Bromine water solution can be prepared in. Bromine In Water Reaction.
From www.slideserve.com
PPT 2.7 Physical Properties of Alkenes PowerPoint Presentation, free Bromine In Water Reaction The products for addition of halogen to alkenes seems straightforward, with each halogen added to each double bond carbon. The presence of the c=c double bond allows alkenes to react in ways that alkanes cannot; When bromine is added to the sample, if the reddish color disappear, that means the sample does contain an alkene. Alkanes and alkenes have different. Bromine In Water Reaction.
From pixels.com
Bromine In Water Photograph by Martyn F. Chillmaid/science Photo Bromine In Water Reaction The addition reaction occurs to get reddish bromine consumed and a. This allows us to tell alkenes apart from alkanes using a simple chemical test called the bromine water test The presence of the c=c double bond allows alkenes to react in ways that alkanes cannot; When bromine is added to the sample, if the reddish color disappear, that means. Bromine In Water Reaction.
From www.sciencephoto.com
Bromine water test for alkenes Stock Image C029/0955 Science Bromine In Water Reaction The reaction with bromine happens at room temperature. When bromine is added to the sample, if the reddish color disappear, that means the sample does contain an alkene. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. The presence of the c=c double bond allows alkenes to react. Bromine In Water Reaction.
From pixels.com
Bromine Water Test Photograph by Martyn F. Chillmaid/science Photo Bromine In Water Reaction Bromine water is a yellow mixture solution with high oxidizing properties, prepared by dissolving diatomic bromine (br 2) in water (h 2 o). Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. The addition reaction occurs to get reddish bromine consumed and colorless product formed, so color fades. Bromine In Water Reaction.
From www.doubtnut.com
Explain the action of bromine water on phenol (carbolic acid). Bromine In Water Reaction Bromine water is a yellow mixture solution with high oxidizing properties, prepared by dissolving diatomic bromine (br 2) in water (h 2 o). The products for addition of halogen to alkenes seems straightforward, with each halogen added to each double bond carbon. All alkanes are saturated and alkenes are unsaturated; The reaction with bromine happens at room temperature. When bromine. Bromine In Water Reaction.
From www.savemyexams.co.uk
Bromine & Alkenes (4.4.2) Edexcel IGCSE Chemistry Revision Notes 2019 Bromine In Water Reaction The reaction with bromine happens at room temperature. When bromine is added to the sample, if the reddish color disappears, it means the sample contains an alkene. The products for addition of halogen to alkenes seems straightforward, with each halogen added to each double bond carbon. Bromine water is a yellow mixture solution with high oxidizing properties, prepared by dissolving. Bromine In Water Reaction.
From www.youtube.com
Bromine Water Experiment YouTube Bromine In Water Reaction The addition reaction occurs to get reddish bromine consumed and colorless product formed, so color fades off. Alkanes and alkenes have different molecular structures; The presence of the c=c double bond allows alkenes to react in ways that alkanes cannot; If you have a gaseous alkene like ethene, you can bubble it through either pure. The products for addition of. Bromine In Water Reaction.
From www.slideserve.com
PPT EXTRACTION OF BROMINE FROM SEA WATER PowerPoint Presentation Bromine In Water Reaction Bromine water is a yellow mixture solution with high oxidizing properties, prepared by dissolving diatomic bromine (br 2) in water (h 2 o). This allows us to tell alkenes apart from alkanes using a simple chemical test called the bromine water test Bromine water solution can be prepared in the chemistry lab by direct mixing of fumes of bromine and. Bromine In Water Reaction.
From ppt-online.org
Addition reactions презентация онлайн Bromine In Water Reaction The presence of the c=c double bond allows alkenes to react in ways that alkanes cannot; When bromine is added to the sample, if the reddish color disappear, that means the sample does contain an alkene. Alkanes and alkenes have different molecular structures; The addition reaction occurs to get reddish bromine consumed and colorless product formed, so color fades off.. Bromine In Water Reaction.
From www.youtube.com
Bromine Water + Sodium Chloride YouTube Bromine In Water Reaction Alkanes and alkenes have different molecular structures; The products for addition of halogen to alkenes seems straightforward, with each halogen added to each double bond carbon. The reaction with bromine happens at room temperature. When bromine is added to the sample, if the reddish color disappears, it means the sample contains an alkene. Bromine water is a yellow mixture solution. Bromine In Water Reaction.
From scienceready.com.au
Bromine Water Test Science Ready Bromine In Water Reaction Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. The presence of the c=c double bond allows alkenes to react in ways that alkanes cannot; When bromine is added to the sample, if the reddish color disappears, it means the sample contains an alkene. When bromine is added. Bromine In Water Reaction.
From www.slideserve.com
PPT EXTRACTION OF BROMINE FROM SEA WATER PowerPoint Presentation ID Bromine In Water Reaction Bromine water solution can be prepared in the chemistry lab by direct mixing of fumes of bromine and water. This allows us to tell alkenes apart from alkanes using a simple chemical test called the bromine water test All alkanes are saturated and alkenes are unsaturated; When bromine is added to the sample, if the reddish color disappears, it means. Bromine In Water Reaction.
From www.slideserve.com
PPT EXTRACTION OF BROMINE FROM SEA WATER PowerPoint Presentation Bromine In Water Reaction The reaction with bromine happens at room temperature. This allows us to tell alkenes apart from alkanes using a simple chemical test called the bromine water test Bromine water is a yellow mixture solution with high oxidizing properties, prepared by dissolving diatomic bromine (br 2) in water (h 2 o). The addition reaction occurs to get reddish bromine consumed and. Bromine In Water Reaction.
From www.youtube.com
Reactions with Bromine Water YouTube Bromine In Water Reaction Bromine water solution can be prepared in the chemistry lab by direct mixing of fumes of bromine and water. This allows us to tell alkenes apart from alkanes using a simple chemical test called the bromine water test The reaction with bromine happens at room temperature. When bromine is added to the sample, if the reddish color disappear, that means. Bromine In Water Reaction.
From fphoto.photoshelter.com
science chemistry reaction bromine cyclohexene Fundamental Bromine In Water Reaction The addition reaction occurs to get reddish bromine consumed and a. All alkanes are saturated and alkenes are unsaturated; The reaction with bromine happens at room temperature. Bromine water is a yellow mixture solution with high oxidizing properties, prepared by dissolving diatomic bromine (br 2) in water (h 2 o). Alkanes and alkenes have different molecular structures; The products for. Bromine In Water Reaction.
From www.vedantu.com
Reaction of bromine water phenol gives white ppt ofA. o bromophenolB Bromine In Water Reaction The reaction with bromine happens at room temperature. The addition reaction occurs to get reddish bromine consumed and a. The products for addition of halogen to alkenes seems straightforward, with each halogen added to each double bond carbon. If you have a gaseous alkene like ethene, you can bubble it through either pure. The presence of the c=c double bond. Bromine In Water Reaction.
From www.slideserve.com
PPT EXTRACTION OF BROMINE FROM SEA WATER PowerPoint Presentation Bromine In Water Reaction The reaction with bromine happens at room temperature. Alkanes and alkenes have different molecular structures; When bromine is added to the sample, if the reddish color disappears, it means the sample contains an alkene. The addition reaction occurs to get reddish bromine consumed and a. All alkanes are saturated and alkenes are unsaturated; Alkenes react in the cold with pure. Bromine In Water Reaction.
From www.youtube.com
Reaction of Bromine with Phenol YouTube Bromine In Water Reaction If you have a gaseous alkene like ethene, you can bubble it through either pure. This allows us to tell alkenes apart from alkanes using a simple chemical test called the bromine water test The presence of the c=c double bond allows alkenes to react in ways that alkanes cannot; When bromine is added to the sample, if the reddish. Bromine In Water Reaction.
From www.coursehero.com
[Solved] The mechanism shows how a solution of bromine in water reacts Bromine In Water Reaction If you have a gaseous alkene like ethene, you can bubble it through either pure. Bromine water solution can be prepared in the chemistry lab by direct mixing of fumes of bromine and water. Alkanes and alkenes have different molecular structures; When bromine is added to the sample, if the reddish color disappear, that means the sample does contain an. Bromine In Water Reaction.
From www.nagwa.com
Question Video Describing the Reaction of Phenol and Alkenes with Bromine In Water Reaction Bromine water is a yellow mixture solution with high oxidizing properties, prepared by dissolving diatomic bromine (br 2) in water (h 2 o). When bromine is added to the sample, if the reddish color disappears, it means the sample contains an alkene. The products for addition of halogen to alkenes seems straightforward, with each halogen added to each double bond. Bromine In Water Reaction.
From mungfali.com
Ethene And Water Reaction Bromine In Water Reaction All alkanes are saturated and alkenes are unsaturated; Bromine water solution can be prepared in the chemistry lab by direct mixing of fumes of bromine and water. The addition reaction occurs to get reddish bromine consumed and a. Alkanes and alkenes have different molecular structures; The products for addition of halogen to alkenes seems straightforward, with each halogen added to. Bromine In Water Reaction.
From www.slideserve.com
PPT EXTRACTION OF BROMINE FROM SEA WATER PowerPoint Presentation Bromine In Water Reaction When bromine is added to the sample, if the reddish color disappear, that means the sample does contain an alkene. If you have a gaseous alkene like ethene, you can bubble it through either pure. The presence of the c=c double bond allows alkenes to react in ways that alkanes cannot; When bromine is added to the sample, if the. Bromine In Water Reaction.
From www.youtube.com
Neutralization of Bromine Beautiful Reaction YouTube Bromine In Water Reaction The products for addition of halogen to alkenes seems straightforward, with each halogen added to each double bond carbon. When bromine is added to the sample, if the reddish color disappears, it means the sample contains an alkene. Bromine water is a yellow mixture solution with high oxidizing properties, prepared by dissolving diatomic bromine (br 2) in water (h 2. Bromine In Water Reaction.
From www.slideserve.com
PPT Qualitative organic analysis PowerPoint Presentation, free Bromine In Water Reaction The presence of the c=c double bond allows alkenes to react in ways that alkanes cannot; Bromine water solution can be prepared in the chemistry lab by direct mixing of fumes of bromine and water. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. The products for addition. Bromine In Water Reaction.
From www.youtube.com
Does bromine water reacts with alcohol? YouTube Bromine In Water Reaction Bromine water is a yellow mixture solution with high oxidizing properties, prepared by dissolving diatomic bromine (br 2) in water (h 2 o). When bromine is added to the sample, if the reddish color disappears, it means the sample contains an alkene. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic. Bromine In Water Reaction.
From www.sciencephoto.com
Test tube of bromine water Stock Image C029/0952 Science Photo Bromine In Water Reaction The addition reaction occurs to get reddish bromine consumed and a. The products for addition of halogen to alkenes seems straightforward, with each halogen added to each double bond carbon. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. The addition reaction occurs to get reddish bromine consumed. Bromine In Water Reaction.
From fineartamerica.com
Bromine Test For Alkene Photograph by Andrew Lambert Photography Bromine In Water Reaction When bromine is added to the sample, if the reddish color disappear, that means the sample does contain an alkene. The addition reaction occurs to get reddish bromine consumed and a. When bromine is added to the sample, if the reddish color disappears, it means the sample contains an alkene. Alkanes and alkenes have different molecular structures; This allows us. Bromine In Water Reaction.