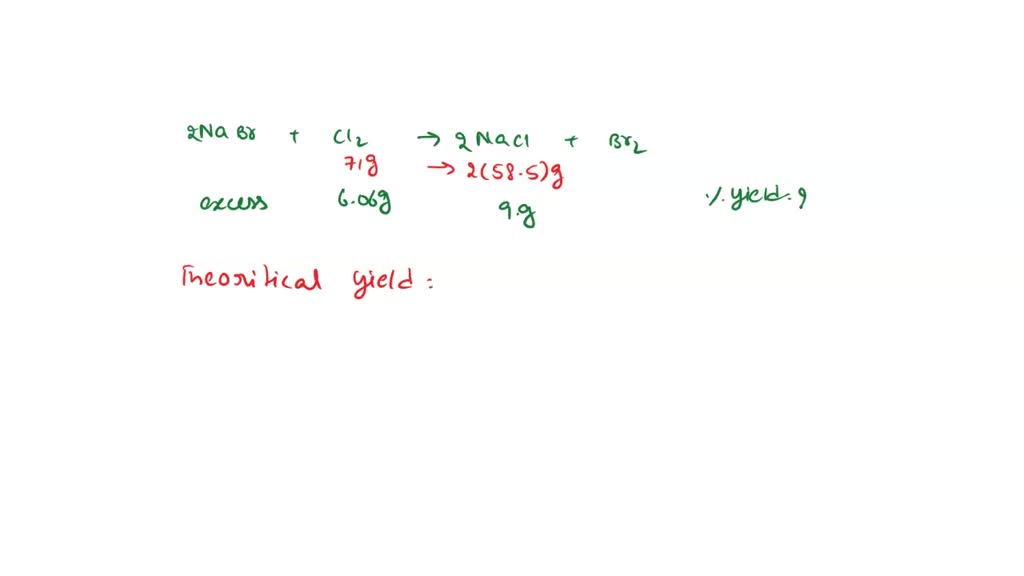Bromine Sodium Chloride Equation . Cl 2 (aq) + 2nabr (aq) → 2nacl (aq) + br 2. Explain the roles of subscripts and coefficients in chemical equations. how to balance nabr + cl2 = nacl + br2 (sodium bromide + chlorine gas) in this video we'll balance the equation. Chlorine + sodium bromide → sodium chloride + bromine. Chlorine + sodium bromide → bromine + sodium. in this equation, the cl and br have swapped places: in another example of a chemical reaction, sodium metal reacts with chlorine gas to form solid sodium chloride. to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. nacl + br = nabr + cl is a single displacement (substitution) reaction where one mole of aqueous sodium chloride. if chlorine is added to a solution of sodium bromide, a reaction occurs:
from www.numerade.com
how to balance nabr + cl2 = nacl + br2 (sodium bromide + chlorine gas) in this video we'll balance the equation. Explain the roles of subscripts and coefficients in chemical equations. Chlorine + sodium bromide → bromine + sodium. nacl + br = nabr + cl is a single displacement (substitution) reaction where one mole of aqueous sodium chloride. to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. in another example of a chemical reaction, sodium metal reacts with chlorine gas to form solid sodium chloride. if chlorine is added to a solution of sodium bromide, a reaction occurs: in this equation, the cl and br have swapped places: Chlorine + sodium bromide → sodium chloride + bromine. Cl 2 (aq) + 2nabr (aq) → 2nacl (aq) + br 2.
SOLVED Given the equation 2 NaBr + Cl2 → Br2 + 2 NaCl 6.06 grams of
Bromine Sodium Chloride Equation in another example of a chemical reaction, sodium metal reacts with chlorine gas to form solid sodium chloride. nacl + br = nabr + cl is a single displacement (substitution) reaction where one mole of aqueous sodium chloride. in another example of a chemical reaction, sodium metal reacts with chlorine gas to form solid sodium chloride. Chlorine + sodium bromide → sodium chloride + bromine. Chlorine + sodium bromide → bromine + sodium. Explain the roles of subscripts and coefficients in chemical equations. if chlorine is added to a solution of sodium bromide, a reaction occurs: how to balance nabr + cl2 = nacl + br2 (sodium bromide + chlorine gas) in this video we'll balance the equation. to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Cl 2 (aq) + 2nabr (aq) → 2nacl (aq) + br 2. in this equation, the cl and br have swapped places:
From www.chegg.com
Solved 3. For the reaction of sodium bromide with chlorine Bromine Sodium Chloride Equation in another example of a chemical reaction, sodium metal reacts with chlorine gas to form solid sodium chloride. in this equation, the cl and br have swapped places: Chlorine + sodium bromide → sodium chloride + bromine. Cl 2 (aq) + 2nabr (aq) → 2nacl (aq) + br 2. Explain the roles of subscripts and coefficients in chemical. Bromine Sodium Chloride Equation.
From www.youtube.com
Reaction of Sodium (Na) with Bromine (Br2) YouTube Bromine Sodium Chloride Equation if chlorine is added to a solution of sodium bromide, a reaction occurs: Chlorine + sodium bromide → sodium chloride + bromine. Chlorine + sodium bromide → bromine + sodium. to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. in another example of a chemical reaction, sodium metal reacts. Bromine Sodium Chloride Equation.
From slideplayer.com
Biology Dr. Altstiel Naples American High School ppt download Bromine Sodium Chloride Equation if chlorine is added to a solution of sodium bromide, a reaction occurs: to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. in another example of a chemical reaction, sodium metal reacts with chlorine gas to form solid sodium chloride. Chlorine + sodium bromide → bromine + sodium. Chlorine. Bromine Sodium Chloride Equation.
From www.youtube.com
Type of Reaction for NaBr + Cl2 = NaCl + Br2 YouTube Bromine Sodium Chloride Equation to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. how to balance nabr + cl2 = nacl + br2 (sodium bromide + chlorine gas) in this video we'll balance the equation. in another example of a chemical reaction, sodium metal reacts with chlorine gas to form solid sodium chloride.. Bromine Sodium Chloride Equation.
From slideplayer.com
Chemical Equations Chapter ppt download Bromine Sodium Chloride Equation in this equation, the cl and br have swapped places: Explain the roles of subscripts and coefficients in chemical equations. if chlorine is added to a solution of sodium bromide, a reaction occurs: to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. how to balance nabr + cl2. Bromine Sodium Chloride Equation.
From dxoayxwhw.blob.core.windows.net
Bromine Sodium Hydroxide Balanced Equation at Lisa Swearengin blog Bromine Sodium Chloride Equation how to balance nabr + cl2 = nacl + br2 (sodium bromide + chlorine gas) in this video we'll balance the equation. in this equation, the cl and br have swapped places: Explain the roles of subscripts and coefficients in chemical equations. if chlorine is added to a solution of sodium bromide, a reaction occurs: Chlorine +. Bromine Sodium Chloride Equation.
From www.numerade.com
SOLVED Sodium chloride reacts with bromine gas, mathrm{Br}_{2}, to Bromine Sodium Chloride Equation Explain the roles of subscripts and coefficients in chemical equations. in another example of a chemical reaction, sodium metal reacts with chlorine gas to form solid sodium chloride. to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Chlorine + sodium bromide → bromine + sodium. Chlorine + sodium bromide →. Bromine Sodium Chloride Equation.
From slideplayer.com
Biology Dr. Altstiel Naples American High School ppt download Bromine Sodium Chloride Equation Chlorine + sodium bromide → sodium chloride + bromine. Chlorine + sodium bromide → bromine + sodium. Cl 2 (aq) + 2nabr (aq) → 2nacl (aq) + br 2. Explain the roles of subscripts and coefficients in chemical equations. in this equation, the cl and br have swapped places: to balance a chemical equation, enter an equation of. Bromine Sodium Chloride Equation.
From www.numerade.com
SOLVED Sodium bromide reacts with calcium chloride to form sodium Bromine Sodium Chloride Equation in this equation, the cl and br have swapped places: in another example of a chemical reaction, sodium metal reacts with chlorine gas to form solid sodium chloride. Chlorine + sodium bromide → sodium chloride + bromine. nacl + br = nabr + cl is a single displacement (substitution) reaction where one mole of aqueous sodium chloride.. Bromine Sodium Chloride Equation.
From www.chegg.com
Solved 9. For the reaction of sodium bromide with chlorine Bromine Sodium Chloride Equation nacl + br = nabr + cl is a single displacement (substitution) reaction where one mole of aqueous sodium chloride. Explain the roles of subscripts and coefficients in chemical equations. in another example of a chemical reaction, sodium metal reacts with chlorine gas to form solid sodium chloride. to balance a chemical equation, enter an equation of. Bromine Sodium Chloride Equation.
From www.numerade.com
SOLVEDBromine can be prepared by adding chlorine to an aqueous Bromine Sodium Chloride Equation how to balance nabr + cl2 = nacl + br2 (sodium bromide + chlorine gas) in this video we'll balance the equation. nacl + br = nabr + cl is a single displacement (substitution) reaction where one mole of aqueous sodium chloride. Chlorine + sodium bromide → bromine + sodium. Cl 2 (aq) + 2nabr (aq) → 2nacl. Bromine Sodium Chloride Equation.
From www.youtube.com
How to Write the Net Ionic Equation for NaBr + Cl2 = NaCl + Br2 YouTube Bromine Sodium Chloride Equation Cl 2 (aq) + 2nabr (aq) → 2nacl (aq) + br 2. in this equation, the cl and br have swapped places: Chlorine + sodium bromide → sodium chloride + bromine. Explain the roles of subscripts and coefficients in chemical equations. Chlorine + sodium bromide → bromine + sodium. how to balance nabr + cl2 = nacl +. Bromine Sodium Chloride Equation.
From ar.inspiredpencil.com
Bromine Liquid Formula Bromine Sodium Chloride Equation if chlorine is added to a solution of sodium bromide, a reaction occurs: nacl + br = nabr + cl is a single displacement (substitution) reaction where one mole of aqueous sodium chloride. to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. how to balance nabr + cl2. Bromine Sodium Chloride Equation.
From www.youtube.com
Chlorine And Sodium Bromide Make Sodium Chloride And Bromine YouTube Bromine Sodium Chloride Equation nacl + br = nabr + cl is a single displacement (substitution) reaction where one mole of aqueous sodium chloride. Cl 2 (aq) + 2nabr (aq) → 2nacl (aq) + br 2. in another example of a chemical reaction, sodium metal reacts with chlorine gas to form solid sodium chloride. to balance a chemical equation, enter an. Bromine Sodium Chloride Equation.
From www.numerade.com
SOLVED 38. Write balanced chemical equation for the following reaction Bromine Sodium Chloride Equation in another example of a chemical reaction, sodium metal reacts with chlorine gas to form solid sodium chloride. Chlorine + sodium bromide → sodium chloride + bromine. to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. nacl + br = nabr + cl is a single displacement (substitution) reaction. Bromine Sodium Chloride Equation.
From www.youtube.com
Bromine Water + Sodium Chloride YouTube Bromine Sodium Chloride Equation in this equation, the cl and br have swapped places: Chlorine + sodium bromide → sodium chloride + bromine. Chlorine + sodium bromide → bromine + sodium. in another example of a chemical reaction, sodium metal reacts with chlorine gas to form solid sodium chloride. Explain the roles of subscripts and coefficients in chemical equations. nacl +. Bromine Sodium Chloride Equation.
From www.numerade.com
SOLVED Chlorine gas reacts with aqueous sodium bromide to produce Bromine Sodium Chloride Equation Cl 2 (aq) + 2nabr (aq) → 2nacl (aq) + br 2. nacl + br = nabr + cl is a single displacement (substitution) reaction where one mole of aqueous sodium chloride. in another example of a chemical reaction, sodium metal reacts with chlorine gas to form solid sodium chloride. Explain the roles of subscripts and coefficients in. Bromine Sodium Chloride Equation.
From dxovypfff.blob.core.windows.net
Aluminum Bromide And Chlorine Gas Formula at Ashley Larkin blog Bromine Sodium Chloride Equation Explain the roles of subscripts and coefficients in chemical equations. to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. in this equation, the cl and br have swapped places: Cl 2 (aq) + 2nabr (aq) → 2nacl (aq) + br 2. if chlorine is added to a solution of. Bromine Sodium Chloride Equation.
From exozgkrga.blob.core.windows.net
HydrideBromide Chemical Formula at Kevin Benton blog Bromine Sodium Chloride Equation to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Chlorine + sodium bromide → bromine + sodium. nacl + br = nabr + cl is a single displacement (substitution) reaction where one mole of aqueous sodium chloride. in this equation, the cl and br have swapped places: Chlorine +. Bromine Sodium Chloride Equation.
From www.numerade.com
SOLVED Given the equation 2 NaBr + Cl2 → Br2 + 2 NaCl 6.06 grams of Bromine Sodium Chloride Equation how to balance nabr + cl2 = nacl + br2 (sodium bromide + chlorine gas) in this video we'll balance the equation. Cl 2 (aq) + 2nabr (aq) → 2nacl (aq) + br 2. Chlorine + sodium bromide → bromine + sodium. in this equation, the cl and br have swapped places: Explain the roles of subscripts and. Bromine Sodium Chloride Equation.
From exovqrrdq.blob.core.windows.net
Bromine And Bromide Formula at James Mota blog Bromine Sodium Chloride Equation Cl 2 (aq) + 2nabr (aq) → 2nacl (aq) + br 2. Chlorine + sodium bromide → bromine + sodium. to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. in another example of a chemical reaction, sodium metal reacts with chlorine gas to form solid sodium chloride. if chlorine. Bromine Sodium Chloride Equation.
From www.numerade.com
SOLVED Aqueous sodium chloride (NaCl) and liquid bromine (Brz are Bromine Sodium Chloride Equation if chlorine is added to a solution of sodium bromide, a reaction occurs: to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. in another example of a chemical reaction, sodium metal reacts with chlorine gas to form solid sodium chloride. how to balance nabr + cl2 = nacl. Bromine Sodium Chloride Equation.
From www.numerade.com
SOLVEDThe nonspontaneous redox reaction of bromine and aqueous sodium Bromine Sodium Chloride Equation how to balance nabr + cl2 = nacl + br2 (sodium bromide + chlorine gas) in this video we'll balance the equation. Cl 2 (aq) + 2nabr (aq) → 2nacl (aq) + br 2. Chlorine + sodium bromide → sodium chloride + bromine. nacl + br = nabr + cl is a single displacement (substitution) reaction where one. Bromine Sodium Chloride Equation.
From wisc.pb.unizin.org
Resonance Structures and Formal Charge (M8Q3) UWMadison Chemistry Bromine Sodium Chloride Equation nacl + br = nabr + cl is a single displacement (substitution) reaction where one mole of aqueous sodium chloride. if chlorine is added to a solution of sodium bromide, a reaction occurs: in another example of a chemical reaction, sodium metal reacts with chlorine gas to form solid sodium chloride. Explain the roles of subscripts and. Bromine Sodium Chloride Equation.
From www.numerade.com
SOLVEDGaseous chlorine will displace bromide ion from an aqueous Bromine Sodium Chloride Equation how to balance nabr + cl2 = nacl + br2 (sodium bromide + chlorine gas) in this video we'll balance the equation. Explain the roles of subscripts and coefficients in chemical equations. nacl + br = nabr + cl is a single displacement (substitution) reaction where one mole of aqueous sodium chloride. in another example of a. Bromine Sodium Chloride Equation.
From slideplayer.com
Chemical Equations & Reactions ppt download Bromine Sodium Chloride Equation Chlorine + sodium bromide → bromine + sodium. Cl 2 (aq) + 2nabr (aq) → 2nacl (aq) + br 2. nacl + br = nabr + cl is a single displacement (substitution) reaction where one mole of aqueous sodium chloride. to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. . Bromine Sodium Chloride Equation.
From www.numerade.com
When chlorine gas is bubbled into a solution of sodium bromide, the Bromine Sodium Chloride Equation to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. in another example of a chemical reaction, sodium metal reacts with chlorine gas to form solid sodium chloride. nacl + br = nabr + cl is a single displacement (substitution) reaction where one mole of aqueous sodium chloride. Chlorine +. Bromine Sodium Chloride Equation.
From brainly.com
Chlorine gas reacts with aqueous sodium bromide to produce aqueous Bromine Sodium Chloride Equation nacl + br = nabr + cl is a single displacement (substitution) reaction where one mole of aqueous sodium chloride. to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. in another example of a chemical reaction, sodium metal reacts with chlorine gas to form solid sodium chloride. Chlorine +. Bromine Sodium Chloride Equation.
From www.numerade.com
SOLVED Word equation and balanced equation for aqueous magnesium Bromine Sodium Chloride Equation Explain the roles of subscripts and coefficients in chemical equations. to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. if chlorine is added to a solution of sodium bromide, a reaction occurs: in another example of a chemical reaction, sodium metal reacts with chlorine gas to form solid sodium. Bromine Sodium Chloride Equation.
From www.numerade.com
SOLVEDWrite a formula for the ionic compound formed from each pair of Bromine Sodium Chloride Equation Explain the roles of subscripts and coefficients in chemical equations. Chlorine + sodium bromide → sodium chloride + bromine. Chlorine + sodium bromide → bromine + sodium. in this equation, the cl and br have swapped places: if chlorine is added to a solution of sodium bromide, a reaction occurs: nacl + br = nabr + cl. Bromine Sodium Chloride Equation.
From www.numerade.com
SOLVED Bromine displaces iodine from sodium iodide, but there is no Bromine Sodium Chloride Equation in another example of a chemical reaction, sodium metal reacts with chlorine gas to form solid sodium chloride. Cl 2 (aq) + 2nabr (aq) → 2nacl (aq) + br 2. Chlorine + sodium bromide → bromine + sodium. in this equation, the cl and br have swapped places: how to balance nabr + cl2 = nacl +. Bromine Sodium Chloride Equation.
From www.youtube.com
NaBr+Cl2=NaCl+Br2 Balanced EquationSodium Bromide+Chlorine=Sodium Bromine Sodium Chloride Equation if chlorine is added to a solution of sodium bromide, a reaction occurs: Explain the roles of subscripts and coefficients in chemical equations. in another example of a chemical reaction, sodium metal reacts with chlorine gas to form solid sodium chloride. Chlorine + sodium bromide → bromine + sodium. Chlorine + sodium bromide → sodium chloride + bromine.. Bromine Sodium Chloride Equation.
From www.chegg.com
Solved 9. For the reaction of sodium bromide with chlorine Bromine Sodium Chloride Equation how to balance nabr + cl2 = nacl + br2 (sodium bromide + chlorine gas) in this video we'll balance the equation. Explain the roles of subscripts and coefficients in chemical equations. to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. nacl + br = nabr + cl is. Bromine Sodium Chloride Equation.
From dxoayxwhw.blob.core.windows.net
Bromine Sodium Hydroxide Balanced Equation at Lisa Swearengin blog Bromine Sodium Chloride Equation in another example of a chemical reaction, sodium metal reacts with chlorine gas to form solid sodium chloride. Explain the roles of subscripts and coefficients in chemical equations. nacl + br = nabr + cl is a single displacement (substitution) reaction where one mole of aqueous sodium chloride. Chlorine + sodium bromide → sodium chloride + bromine. Cl. Bromine Sodium Chloride Equation.
From www.youtube.com
How to Balance NaBr + Cl2 = NaCl + Br2 (Sodium Bromide + Chlorine gas Bromine Sodium Chloride Equation in this equation, the cl and br have swapped places: Chlorine + sodium bromide → sodium chloride + bromine. how to balance nabr + cl2 = nacl + br2 (sodium bromide + chlorine gas) in this video we'll balance the equation. if chlorine is added to a solution of sodium bromide, a reaction occurs: nacl +. Bromine Sodium Chloride Equation.