Calorimetry Introduction . This page briefly introduces the measuring principle of calorimetry, and the method of calorimetry. Calorimetry literally means “heat measurement.” for consistency with other forms of energy and to avoid confusion, the modern unit for. A system is the specific place where a reaction or process is occurring (eg inside calorimeter). The surroundings are outside of the system (eg the lab. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. At last, this page prospects the quantity method and. For example, when an exothermic reaction occurs in solution in a calorimeter, the heat. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process.
from bitesizebio.com
The surroundings are outside of the system (eg the lab. Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry literally means “heat measurement.” for consistency with other forms of energy and to avoid confusion, the modern unit for. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. This page briefly introduces the measuring principle of calorimetry, and the method of calorimetry. For example, when an exothermic reaction occurs in solution in a calorimeter, the heat. At last, this page prospects the quantity method and. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. A system is the specific place where a reaction or process is occurring (eg inside calorimeter).
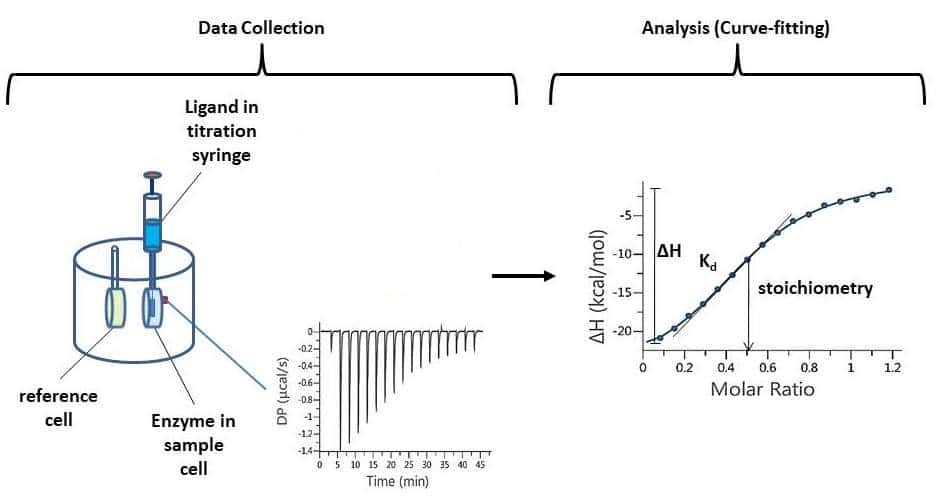
How Strong is Your Binding? A Quick Introduction to Isothermal
Calorimetry Introduction Calorimetry literally means “heat measurement.” for consistency with other forms of energy and to avoid confusion, the modern unit for. The surroundings are outside of the system (eg the lab. At last, this page prospects the quantity method and. A system is the specific place where a reaction or process is occurring (eg inside calorimeter). To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry literally means “heat measurement.” for consistency with other forms of energy and to avoid confusion, the modern unit for. For example, when an exothermic reaction occurs in solution in a calorimeter, the heat. This page briefly introduces the measuring principle of calorimetry, and the method of calorimetry. Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes.
From www.chegg.com
Solved CALORIMETRY HEAT CAPACITY OF A CALORIMETER Calorimetry Introduction Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. This page briefly introduces the measuring principle of calorimetry, and the method of calorimetry. A system is the specific place where a reaction or process is occurring (eg inside calorimeter). The surroundings are outside of the system (eg. Calorimetry Introduction.
From www.studocu.com
Calorimetry Lab Report S Calorimetry Introduction In this experiment Calorimetry Introduction For example, when an exothermic reaction occurs in solution in a calorimeter, the heat. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Calorimetry is the measurement of the transfer. Calorimetry Introduction.
From studylib.net
LAB 1 CALORIMETRY OBJECTIVES 1. Introduction to the Calorimetry Introduction This page briefly introduces the measuring principle of calorimetry, and the method of calorimetry. The surroundings are outside of the system (eg the lab. Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. Calorimetry is a branch of science concerned with measuring a body’s state in terms. Calorimetry Introduction.
From www.numerade.com
SOLVED hello can someone please help? im not sure why its marking Calorimetry Introduction The surroundings are outside of the system (eg the lab. Calorimetry literally means “heat measurement.” for consistency with other forms of energy and to avoid confusion, the modern unit for. At last, this page prospects the quantity method and. For example, when an exothermic reaction occurs in solution in a calorimeter, the heat. This page briefly introduces the measuring principle. Calorimetry Introduction.
From faqguide.co
What does a calorimeter do? Explained by FAQGuide Calorimetry Introduction For example, when an exothermic reaction occurs in solution in a calorimeter, the heat. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process.. Calorimetry Introduction.
From www.scribd.com
Lab 4 Calorimetry Lab PDF Temperature Heat Capacity Calorimetry Introduction For example, when an exothermic reaction occurs in solution in a calorimeter, the heat. Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. The surroundings are outside of the system (eg the lab. This page briefly introduces the measuring principle of calorimetry, and the method of calorimetry.. Calorimetry Introduction.
From www.studocu.com
Calorimeter Lab lab report Calorimetry Introduction The purpose of Calorimetry Introduction Calorimetry literally means “heat measurement.” for consistency with other forms of energy and to avoid confusion, the modern unit for. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. A calorimeter is. Calorimetry Introduction.
From mechasource.blogspot.com
An Introduction To Calorimetry types And Uses , Bomb and Boy,s Gas Calorimetry Introduction A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The surroundings are outside of the system (eg the lab. At last, this page prospects the quantity method and. This page briefly introduces the measuring principle of calorimetry, and the method of calorimetry. To determine the enthalpy, stability, heat capacity, and. Calorimetry Introduction.
From www.youtube.com
Bomb Calorimetry Introduction Physical Chemistry Laboratory YouTube Calorimetry Introduction To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Calorimetry literally means “heat measurement.” for consistency with other forms of energy and to avoid confusion, the modern unit for. Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. At last, this. Calorimetry Introduction.
From studylib.net
252b Lecture 8 Calorimetry Calorimetry Introduction Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. For example, when an exothermic reaction occurs in solution in a calorimeter, the heat. This page briefly introduces the measuring principle of calorimetry,. Calorimetry Introduction.
From www.slideserve.com
PPT CALORIMETRY PowerPoint Presentation, free download ID9399695 Calorimetry Introduction At last, this page prospects the quantity method and. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. The surroundings are outside of the system (eg the lab. A system is the specific place where a reaction or process is occurring (eg inside calorimeter). Calorimetry is a branch of science concerned with measuring a. Calorimetry Introduction.
From www.studocu.com
Chem 112 Calorimetry Pdf CALORIMETRY INTRODUCTION The heat evolved Calorimetry Introduction Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. A system is the specific place where a reaction or process is occurring (eg inside calorimeter). This page briefly introduces the. Calorimetry Introduction.
From mechasource.blogspot.com
An Introduction To Calorimetry types And Uses , Bomb and Boy,s Gas Calorimetry Introduction A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. At last, this page prospects the quantity method and. For example, when an exothermic reaction occurs in solution in. Calorimetry Introduction.
From www.degruyter.com
Thermal Analysis and Calorimetry Calorimetry Introduction At last, this page prospects the quantity method and. A system is the specific place where a reaction or process is occurring (eg inside calorimeter). Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. This page briefly introduces the measuring principle of calorimetry, and the. Calorimetry Introduction.
From www.slideserve.com
PPT CALORIMETRY PowerPoint Presentation, free download ID5767247 Calorimetry Introduction This page briefly introduces the measuring principle of calorimetry, and the method of calorimetry. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. The surroundings are. Calorimetry Introduction.
From www.chegg.com
Solved Pre Lab for Calorimetry Introduction Calorimetry is Calorimetry Introduction Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Calorimetry literally means “heat measurement.” for consistency with other forms of energy and to avoid confusion, the modern unit for. Calorimetry is the measurement of the transfer of heat into or out of a system during. Calorimetry Introduction.
From bitesizebio.com
How Strong is Your Binding? A Quick Introduction to Isothermal Calorimetry Introduction This page briefly introduces the measuring principle of calorimetry, and the method of calorimetry. Calorimetry literally means “heat measurement.” for consistency with other forms of energy and to avoid confusion, the modern unit for. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. At last,. Calorimetry Introduction.
From www.studocu.com
Thermochemistry and Calorimetry Measuring Heat Changes in Chemical Calorimetry Introduction To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. A system is the specific place where a reaction or process is occurring (eg inside calorimeter). Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Calorimetry literally means “heat measurement.”. Calorimetry Introduction.
From www.studocu.com
Calorimetry Lab Report Write an abstract for the calorimetry Calorimetry Introduction For example, when an exothermic reaction occurs in solution in a calorimeter, the heat. The surroundings are outside of the system (eg the lab. Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. At last, this page prospects the quantity method and. This page briefly introduces the. Calorimetry Introduction.
From www.slideserve.com
PPT Differential Scanning Calorimetry PowerPoint Presentation, free Calorimetry Introduction A system is the specific place where a reaction or process is occurring (eg inside calorimeter). At last, this page prospects the quantity method and. This page briefly introduces the measuring principle of calorimetry, and the method of calorimetry. For example, when an exothermic reaction occurs in solution in a calorimeter, the heat. A calorimeter is a device used to. Calorimetry Introduction.
From www.docsity.com
Calorimetry lab report Study Guides, Projects, Research Chemistry Calorimetry Introduction Calorimetry literally means “heat measurement.” for consistency with other forms of energy and to avoid confusion, the modern unit for. A system is the specific place where a reaction or process is occurring (eg inside calorimeter). Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. A calorimeter. Calorimetry Introduction.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6133898 Calorimetry Introduction Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. The surroundings are outside of the system (eg the lab. At last, this page prospects the quantity method and. A system is the specific place where a reaction or process is occurring (eg inside calorimeter). For. Calorimetry Introduction.
From www.youtube.com
Thermochemistry Calorimetry Introduction YouTube Calorimetry Introduction This page briefly introduces the measuring principle of calorimetry, and the method of calorimetry. A system is the specific place where a reaction or process is occurring (eg inside calorimeter). The surroundings are outside of the system (eg the lab. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Calorimetry literally means “heat measurement.”. Calorimetry Introduction.
From studylib.net
Calorimetry_and_Specific_Heat_Capacity Calorimetry Introduction For example, when an exothermic reaction occurs in solution in a calorimeter, the heat. Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. The surroundings are outside of the system (eg the lab. A system is the specific place where a reaction or process is occurring (eg. Calorimetry Introduction.
From en.ppt-online.org
What is enthalpy? online presentation Calorimetry Introduction This page briefly introduces the measuring principle of calorimetry, and the method of calorimetry. For example, when an exothermic reaction occurs in solution in a calorimeter, the heat. A system is the specific place where a reaction or process is occurring (eg inside calorimeter). A calorimeter is a device used to measure the amount of heat involved in a chemical. Calorimetry Introduction.
From www.studocu.com
Calorimetry Lab Chem 112 General Chemistry StuDocu Calorimetry Introduction A system is the specific place where a reaction or process is occurring (eg inside calorimeter). Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. For example, when an exothermic reaction occurs in solution in a calorimeter, the heat. The surroundings are outside of the. Calorimetry Introduction.
From www.linstitute.net
Edexcel A Level Chemistry复习笔记1.8.3 Calorimetry翰林国际教育 Calorimetry Introduction Calorimetry literally means “heat measurement.” for consistency with other forms of energy and to avoid confusion, the modern unit for. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. At last, this page prospects the quantity method and. The surroundings are outside of the system. Calorimetry Introduction.
From www.youtube.com
Introduction to Differential Scanning Calorimetry YouTube Calorimetry Introduction A system is the specific place where a reaction or process is occurring (eg inside calorimeter). Calorimetry literally means “heat measurement.” for consistency with other forms of energy and to avoid confusion, the modern unit for. For example, when an exothermic reaction occurs in solution in a calorimeter, the heat. This page briefly introduces the measuring principle of calorimetry, and. Calorimetry Introduction.
From www.chegg.com
Solved = EXP 11 CALORIMETRY. HEAT CAPACITY OF A Calorimetry Introduction This page briefly introduces the measuring principle of calorimetry, and the method of calorimetry. Calorimetry literally means “heat measurement.” for consistency with other forms of energy and to avoid confusion, the modern unit for. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is the measurement of the transfer. Calorimetry Introduction.
From www.collegesearch.in
Principle of Calorimetry Definition, Formula, Principle, Types Calorimetry Introduction To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. A system is the specific place where a reaction or process is occurring (eg inside calorimeter). Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Calorimetry is the measurement of. Calorimetry Introduction.
From www.slideserve.com
PPT Calorimetry C ontents Basic Concept Example Whiteboards Bomb Calorimetry Introduction Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. Calorimetry literally means “heat measurement.” for consistency with other forms of energy and to avoid confusion, the modern unit for. A system is the specific place where a reaction or process is occurring (eg inside calorimeter). To determine. Calorimetry Introduction.
From www.studocu.com
Calorimetry Lab Report Experiment 6. Calorimetry Purpose The purpose Calorimetry Introduction Calorimetry literally means “heat measurement.” for consistency with other forms of energy and to avoid confusion, the modern unit for. For example, when an exothermic reaction occurs in solution in a calorimeter, the heat. At last, this page prospects the quantity method and. A system is the specific place where a reaction or process is occurring (eg inside calorimeter). A. Calorimetry Introduction.
From www.slideserve.com
PPT Introduction to Thermochemistry PowerPoint Presentation, free Calorimetry Introduction At last, this page prospects the quantity method and. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry literally means “heat measurement.” for consistency with other forms of energy and to avoid confusion, the modern unit for. Calorimetry is the measurement of the transfer of heat into or out. Calorimetry Introduction.
From www.coursehero.com
[Solved] LAB 9 CALORIMETRY PRELAB 9 CALORIMETRY SIMULATIONS Calorimetry Introduction This page briefly introduces the measuring principle of calorimetry, and the method of calorimetry. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. A system is the specific place where a reaction or process is occurring (eg inside calorimeter). The surroundings are outside of the. Calorimetry Introduction.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Calorimetry Introduction This page briefly introduces the measuring principle of calorimetry, and the method of calorimetry. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. At last, this page prospects the quantity method and. Calorimetry literally means “heat measurement.” for consistency with other forms of energy and to avoid confusion, the modern unit for. A system. Calorimetry Introduction.