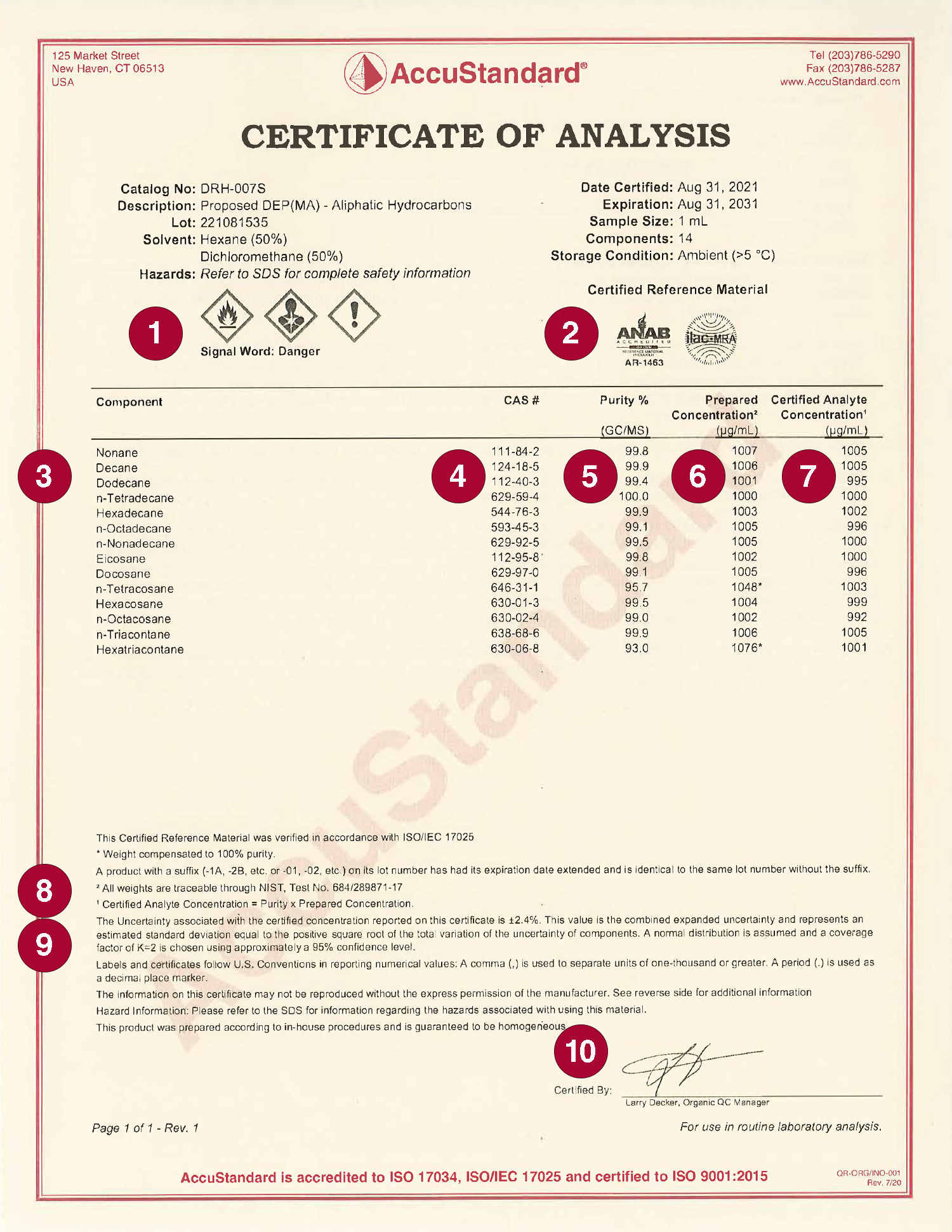
Packing Material Coa Format . The commonly used packaging materials are container, closure, carton or outer and box. The coa’s of raw materials and packaging materials shall contain the following specified information. 5/5 (75) Manufacturers, fabricators, importers and/or distributors of approved packaging materials shall provide. Packaging materials for pharmaceutical products. The containers may be made of glass,. The document attests that the product has undergone extensive testing in a certified lab. 4,5/5 (129) 4,5/5 (129) Certificates of analysis (coa) have to be issued for excipients, apis, packaging materials and finished products. The name (chemical name or. To provide a standard operating procedure for sampling of packaging materials (pm) in pharmaceuticals. Coa for raw and packaging material. Free certificate of analysis templates (guide & examples) the certificate of analysis is a legally binding document that is issued by a certification authority regarding a product. In our gmp series packaging materials for.
from mungfali.com
4,5/5 (129) Packaging materials for pharmaceutical products. The name (chemical name or. The containers may be made of glass,. The commonly used packaging materials are container, closure, carton or outer and box. To provide a standard operating procedure for sampling of packaging materials (pm) in pharmaceuticals. The document attests that the product has undergone extensive testing in a certified lab. Manufacturers, fabricators, importers and/or distributors of approved packaging materials shall provide. Coa for raw and packaging material. In our gmp series packaging materials for.
Certificate Of Analysis COA Template
Packing Material Coa Format Free certificate of analysis templates (guide & examples) the certificate of analysis is a legally binding document that is issued by a certification authority regarding a product. The coa’s of raw materials and packaging materials shall contain the following specified information. To provide a standard operating procedure for sampling of packaging materials (pm) in pharmaceuticals. In our gmp series packaging materials for. The containers may be made of glass,. Free certificate of analysis templates (guide & examples) the certificate of analysis is a legally binding document that is issued by a certification authority regarding a product. Packaging materials for pharmaceutical products. 4,5/5 (129) The name (chemical name or. 4,5/5 (129) Coa for raw and packaging material. Certificates of analysis (coa) have to be issued for excipients, apis, packaging materials and finished products. The document attests that the product has undergone extensive testing in a certified lab. Manufacturers, fabricators, importers and/or distributors of approved packaging materials shall provide. 5/5 (75) The commonly used packaging materials are container, closure, carton or outer and box.
From templates.udlvirtual.edu.pe
Free Packing Slip Template Pdf Printable Templates Packing Material Coa Format The document attests that the product has undergone extensive testing in a certified lab. The name (chemical name or. The containers may be made of glass,. Coa for raw and packaging material. Packaging materials for pharmaceutical products. 4,5/5 (129) The commonly used packaging materials are container, closure, carton or outer and box. The coa’s of raw materials and packaging. Packing Material Coa Format.
From medium.com
The Complete Guide to CBD COA (Certificate Of Analysis) by Alphagreen Packing Material Coa Format Coa for raw and packaging material. The coa’s of raw materials and packaging materials shall contain the following specified information. To provide a standard operating procedure for sampling of packaging materials (pm) in pharmaceuticals. 4,5/5 (129) In our gmp series packaging materials for. Certificates of analysis (coa) have to be issued for excipients, apis, packaging materials and finished products.. Packing Material Coa Format.
From www.downunderenterprises.com
Nerolina Down Under Enterprises Packing Material Coa Format To provide a standard operating procedure for sampling of packaging materials (pm) in pharmaceuticals. The containers may be made of glass,. The name (chemical name or. Certificates of analysis (coa) have to be issued for excipients, apis, packaging materials and finished products. Manufacturers, fabricators, importers and/or distributors of approved packaging materials shall provide. Coa for raw and packaging material. 5/5. Packing Material Coa Format.
From eamaterials.com
Elite Advanced Materials Sdn Bhd Quality Control and Why it is Important? Packing Material Coa Format In our gmp series packaging materials for. 5/5 (75) The document attests that the product has undergone extensive testing in a certified lab. To provide a standard operating procedure for sampling of packaging materials (pm) in pharmaceuticals. The containers may be made of glass,. Certificates of analysis (coa) have to be issued for excipients, apis, packaging materials and finished. Packing Material Coa Format.
From www.studocu.com
Tài liệu Logistics Total CTN 2,400 US Shipping document Packing Material Coa Format 5/5 (75) Manufacturers, fabricators, importers and/or distributors of approved packaging materials shall provide. The commonly used packaging materials are container, closure, carton or outer and box. 4,5/5 (129) The coa’s of raw materials and packaging materials shall contain the following specified information. The containers may be made of glass,. To provide a standard operating procedure for sampling of. Packing Material Coa Format.
From infinitecal.com
Understanding a Certificate of Analysis (COA) Infinite Chemical Packing Material Coa Format 4,5/5 (129) Coa for raw and packaging material. 4,5/5 (129) The name (chemical name or. In our gmp series packaging materials for. Packaging materials for pharmaceutical products. The commonly used packaging materials are container, closure, carton or outer and box. The coa’s of raw materials and packaging materials shall contain the following specified information. Free certificate of analysis. Packing Material Coa Format.
From www.scribd.com
Packaging Material Specification Sheet PDF Specification (Technical Packing Material Coa Format Packaging materials for pharmaceutical products. In our gmp series packaging materials for. The document attests that the product has undergone extensive testing in a certified lab. Coa for raw and packaging material. Manufacturers, fabricators, importers and/or distributors of approved packaging materials shall provide. 5/5 (75) The coa’s of raw materials and packaging materials shall contain the following specified information.. Packing Material Coa Format.
From www.cloudtenlab.com
Example COA Cloud TEN Packing Material Coa Format The containers may be made of glass,. The document attests that the product has undergone extensive testing in a certified lab. Free certificate of analysis templates (guide & examples) the certificate of analysis is a legally binding document that is issued by a certification authority regarding a product. To provide a standard operating procedure for sampling of packaging materials (pm). Packing Material Coa Format.
From medium.com
The Complete Guide to CBD COA (Certificate Of Analysis) by Alphagreen Packing Material Coa Format The containers may be made of glass,. Packaging materials for pharmaceutical products. The coa’s of raw materials and packaging materials shall contain the following specified information. Free certificate of analysis templates (guide & examples) the certificate of analysis is a legally binding document that is issued by a certification authority regarding a product. The name (chemical name or. To provide. Packing Material Coa Format.
From www.datacor.com
2024 Certificate of Analysis Definition, Example Template & Requirements Packing Material Coa Format Manufacturers, fabricators, importers and/or distributors of approved packaging materials shall provide. The name (chemical name or. In our gmp series packaging materials for. Free certificate of analysis templates (guide & examples) the certificate of analysis is a legally binding document that is issued by a certification authority regarding a product. The coa’s of raw materials and packaging materials shall contain. Packing Material Coa Format.
From packaging.shiprocket.in
Types of Packaging for Pharmaceutical Products · SHIPROCKET PRIVATE LIMITED Packing Material Coa Format The coa’s of raw materials and packaging materials shall contain the following specified information. The document attests that the product has undergone extensive testing in a certified lab. The containers may be made of glass,. 5/5 (75) 4,5/5 (129) In our gmp series packaging materials for. Free certificate of analysis templates (guide & examples) the certificate of analysis. Packing Material Coa Format.
From ambercourier.com
What is Packing Declaration? Amber Courier Packing Material Coa Format 4,5/5 (129) The containers may be made of glass,. Manufacturers, fabricators, importers and/or distributors of approved packaging materials shall provide. In our gmp series packaging materials for. 5/5 (75) The name (chemical name or. Free certificate of analysis templates (guide & examples) the certificate of analysis is a legally binding document that is issued by a certification authority. Packing Material Coa Format.
From www.scribd.com
COA Finished Product Packing Material Coa Format Free certificate of analysis templates (guide & examples) the certificate of analysis is a legally binding document that is issued by a certification authority regarding a product. Coa for raw and packaging material. 4,5/5 (129) The containers may be made of glass,. The name (chemical name or. Manufacturers, fabricators, importers and/or distributors of approved packaging materials shall provide. In. Packing Material Coa Format.
From chylocure.com
COA Directory ChyloCure Packing Material Coa Format To provide a standard operating procedure for sampling of packaging materials (pm) in pharmaceuticals. In our gmp series packaging materials for. Manufacturers, fabricators, importers and/or distributors of approved packaging materials shall provide. 4,5/5 (129) Free certificate of analysis templates (guide & examples) the certificate of analysis is a legally binding document that is issued by a certification authority regarding. Packing Material Coa Format.
From www.scribd.com
Coa Format PDF Packing Material Coa Format Coa for raw and packaging material. In our gmp series packaging materials for. The name (chemical name or. The coa’s of raw materials and packaging materials shall contain the following specified information. To provide a standard operating procedure for sampling of packaging materials (pm) in pharmaceuticals. The containers may be made of glass,. The commonly used packaging materials are container,. Packing Material Coa Format.
From www.scribd.com
Seaworthy Packing Procedure Packaging And Labeling Structural Steel Packing Material Coa Format In our gmp series packaging materials for. The coa’s of raw materials and packaging materials shall contain the following specified information. 4,5/5 (129) Free certificate of analysis templates (guide & examples) the certificate of analysis is a legally binding document that is issued by a certification authority regarding a product. The commonly used packaging materials are container, closure, carton. Packing Material Coa Format.
From www.symteraanalytics.com
Sample CoA Packing Material Coa Format The name (chemical name or. The document attests that the product has undergone extensive testing in a certified lab. Certificates of analysis (coa) have to be issued for excipients, apis, packaging materials and finished products. The containers may be made of glass,. To provide a standard operating procedure for sampling of packaging materials (pm) in pharmaceuticals. Manufacturers, fabricators, importers and/or. Packing Material Coa Format.
From www.scribd.com
Packing ListCoa PDF Packing Material Coa Format Certificates of analysis (coa) have to be issued for excipients, apis, packaging materials and finished products. The coa’s of raw materials and packaging materials shall contain the following specified information. The document attests that the product has undergone extensive testing in a certified lab. The name (chemical name or. Free certificate of analysis templates (guide & examples) the certificate of. Packing Material Coa Format.
From www.foodtechnetwork.in
Labelling Display and Packaging Materials Regulations of FSSAI Packing Material Coa Format Certificates of analysis (coa) have to be issued for excipients, apis, packaging materials and finished products. Free certificate of analysis templates (guide & examples) the certificate of analysis is a legally binding document that is issued by a certification authority regarding a product. To provide a standard operating procedure for sampling of packaging materials (pm) in pharmaceuticals. The commonly used. Packing Material Coa Format.
From www.youtube.com
Corrugated Box Specification Sheet Corrugated box Part17 YouTube Packing Material Coa Format 4,5/5 (129) Free certificate of analysis templates (guide & examples) the certificate of analysis is a legally binding document that is issued by a certification authority regarding a product. To provide a standard operating procedure for sampling of packaging materials (pm) in pharmaceuticals. 5/5 (75) The document attests that the product has undergone extensive testing in a certified. Packing Material Coa Format.
From www.samplestemplates.org
14+ Free Packing List Templates Free Formats Excel Word Packing Material Coa Format Manufacturers, fabricators, importers and/or distributors of approved packaging materials shall provide. 4,5/5 (129) In our gmp series packaging materials for. Coa for raw and packaging material. To provide a standard operating procedure for sampling of packaging materials (pm) in pharmaceuticals. The coa’s of raw materials and packaging materials shall contain the following specified information. The containers may be made. Packing Material Coa Format.
From www.pinterest.ph
Example of a packing declaration document stating ISPM15 and types of Packing Material Coa Format Free certificate of analysis templates (guide & examples) the certificate of analysis is a legally binding document that is issued by a certification authority regarding a product. The coa’s of raw materials and packaging materials shall contain the following specified information. The commonly used packaging materials are container, closure, carton or outer and box. Manufacturers, fabricators, importers and/or distributors of. Packing Material Coa Format.
From www.wordtemplatesonline.net
Free Certificate of Analysis Templates (Guide & Examples) Packing Material Coa Format The document attests that the product has undergone extensive testing in a certified lab. 4,5/5 (129) 4,5/5 (129) The containers may be made of glass,. Free certificate of analysis templates (guide & examples) the certificate of analysis is a legally binding document that is issued by a certification authority regarding a product. 5/5 (75) Manufacturers, fabricators, importers. Packing Material Coa Format.
From homecare24.id
Coa Produk Adalah Homecare24 Packing Material Coa Format In our gmp series packaging materials for. 4,5/5 (129) The containers may be made of glass,. The coa’s of raw materials and packaging materials shall contain the following specified information. 5/5 (75) Free certificate of analysis templates (guide & examples) the certificate of analysis is a legally binding document that is issued by a certification authority regarding a. Packing Material Coa Format.
From www.kinnogold.com
COA Packing Material Coa Format The document attests that the product has undergone extensive testing in a certified lab. To provide a standard operating procedure for sampling of packaging materials (pm) in pharmaceuticals. The name (chemical name or. The coa’s of raw materials and packaging materials shall contain the following specified information. In our gmp series packaging materials for. 4,5/5 (129) The commonly used. Packing Material Coa Format.
From www.scribd.com
Coa Format Packaging PDF Packing Material Coa Format Coa for raw and packaging material. Certificates of analysis (coa) have to be issued for excipients, apis, packaging materials and finished products. The name (chemical name or. To provide a standard operating procedure for sampling of packaging materials (pm) in pharmaceuticals. Free certificate of analysis templates (guide & examples) the certificate of analysis is a legally binding document that is. Packing Material Coa Format.
From mavink.com
Modelo De Packing List Packing Material Coa Format To provide a standard operating procedure for sampling of packaging materials (pm) in pharmaceuticals. Coa for raw and packaging material. In our gmp series packaging materials for. Manufacturers, fabricators, importers and/or distributors of approved packaging materials shall provide. The document attests that the product has undergone extensive testing in a certified lab. Free certificate of analysis templates (guide & examples). Packing Material Coa Format.
From www.scribd.com
COA Format For Besan PDF Foods Materials Packing Material Coa Format 4,5/5 (129) The coa’s of raw materials and packaging materials shall contain the following specified information. Free certificate of analysis templates (guide & examples) the certificate of analysis is a legally binding document that is issued by a certification authority regarding a product. The name (chemical name or. 4,5/5 (129) Coa for raw and packaging material. Certificates of. Packing Material Coa Format.
From www.dohapharma.com
Shipping document Packing Material Coa Format 5/5 (75) The coa’s of raw materials and packaging materials shall contain the following specified information. The document attests that the product has undergone extensive testing in a certified lab. To provide a standard operating procedure for sampling of packaging materials (pm) in pharmaceuticals. The name (chemical name or. Packaging materials for pharmaceutical products. 4,5/5 (129) In our. Packing Material Coa Format.
From www.baweicosmetic.com
Quality Control Guangdong Bawei Biotechnology Corporation. Packing Material Coa Format Coa for raw and packaging material. Manufacturers, fabricators, importers and/or distributors of approved packaging materials shall provide. 4,5/5 (129) Certificates of analysis (coa) have to be issued for excipients, apis, packaging materials and finished products. Packaging materials for pharmaceutical products. The commonly used packaging materials are container, closure, carton or outer and box. The name (chemical name or. Free. Packing Material Coa Format.
From mungfali.com
Certificate Of Analysis COA Template Packing Material Coa Format Manufacturers, fabricators, importers and/or distributors of approved packaging materials shall provide. 4,5/5 (129) Free certificate of analysis templates (guide & examples) the certificate of analysis is a legally binding document that is issued by a certification authority regarding a product. The name (chemical name or. The coa’s of raw materials and packaging materials shall contain the following specified information.. Packing Material Coa Format.
From www.academia.edu
(DOC) Sample COA format Tim tips Academia.edu Packing Material Coa Format Free certificate of analysis templates (guide & examples) the certificate of analysis is a legally binding document that is issued by a certification authority regarding a product. The name (chemical name or. Certificates of analysis (coa) have to be issued for excipients, apis, packaging materials and finished products. 4,5/5 (129) Coa for raw and packaging material. Manufacturers, fabricators, importers. Packing Material Coa Format.
From www.chemicasoft.com
Pistachio The Complete Flavour and Fragrance software solution Packing Material Coa Format To provide a standard operating procedure for sampling of packaging materials (pm) in pharmaceuticals. The commonly used packaging materials are container, closure, carton or outer and box. Manufacturers, fabricators, importers and/or distributors of approved packaging materials shall provide. The containers may be made of glass,. The document attests that the product has undergone extensive testing in a certified lab. 4,5/5. Packing Material Coa Format.
From chemicasoft.com
Pistachio The Complete Flavour and Fragrance software solution Packing Material Coa Format Coa for raw and packaging material. The commonly used packaging materials are container, closure, carton or outer and box. Manufacturers, fabricators, importers and/or distributors of approved packaging materials shall provide. The containers may be made of glass,. The name (chemical name or. Certificates of analysis (coa) have to be issued for excipients, apis, packaging materials and finished products. To provide. Packing Material Coa Format.
From www.thermofisher.com
CTS™ OpTmizer™ T Cell Expansion SFM, bottle format Packing Material Coa Format Manufacturers, fabricators, importers and/or distributors of approved packaging materials shall provide. The name (chemical name or. The coa’s of raw materials and packaging materials shall contain the following specified information. The commonly used packaging materials are container, closure, carton or outer and box. The document attests that the product has undergone extensive testing in a certified lab. 4,5/5 (129). Packing Material Coa Format.