Temperature Change Energy Equation . Heat transfer and temperature change. Explain that temperature is a measure of internal kinetic energy; A practical approximation for the relationship between heat transfer and temperature change is: Heat is driven by temperature differences, while work involves a force exerted through a distance. Multiply the change in temperature with the mass of the sample. Interconvert temperatures between celsius, kelvin, and fahrenheit scales Q = mcδt, where q is the symbol for heat transfer, m is the mass of the substance,. \[q = mc\delta t,\] where \(q\) is. Change in thermal energy = mass × specific heat. The quantitative relationship between heat transfer and temperature change contains all three factors: The change in thermal energy due to temperature changes is calculated using this equation: Nevertheless, heat and work can produce. Subtract the final and initial temperature to get the change in temperature (δt).
from www.youtube.com
Q = mcδt, where q is the symbol for heat transfer, m is the mass of the substance,. Subtract the final and initial temperature to get the change in temperature (δt). Interconvert temperatures between celsius, kelvin, and fahrenheit scales Nevertheless, heat and work can produce. Explain that temperature is a measure of internal kinetic energy; Heat is driven by temperature differences, while work involves a force exerted through a distance. The quantitative relationship between heat transfer and temperature change contains all three factors: \[q = mc\delta t,\] where \(q\) is. A practical approximation for the relationship between heat transfer and temperature change is: Heat transfer and temperature change.
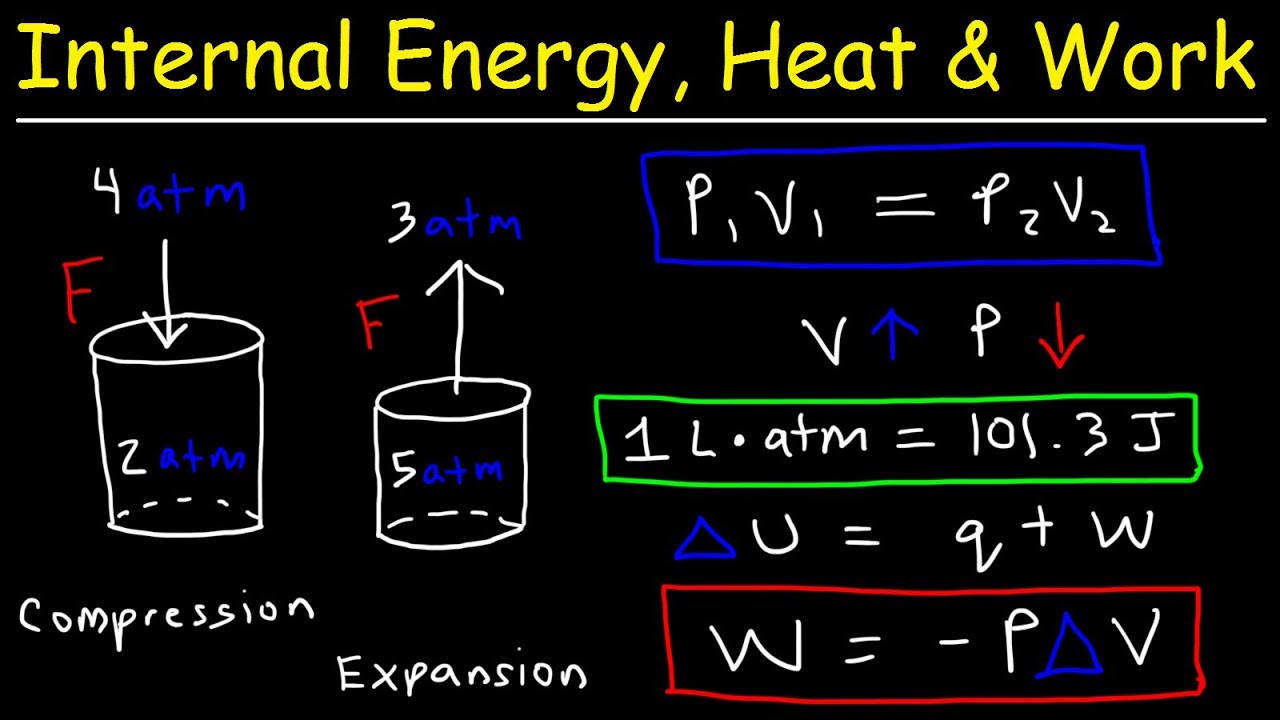
Internal Energy, Heat, and Work Thermodynamics, Pressure & Volume
Temperature Change Energy Equation Nevertheless, heat and work can produce. Multiply the change in temperature with the mass of the sample. Interconvert temperatures between celsius, kelvin, and fahrenheit scales Subtract the final and initial temperature to get the change in temperature (δt). The quantitative relationship between heat transfer and temperature change contains all three factors: Nevertheless, heat and work can produce. Heat is driven by temperature differences, while work involves a force exerted through a distance. Change in thermal energy = mass × specific heat. \[q = mc\delta t,\] where \(q\) is. A practical approximation for the relationship between heat transfer and temperature change is: Explain that temperature is a measure of internal kinetic energy; The change in thermal energy due to temperature changes is calculated using this equation: Q = mcδt, where q is the symbol for heat transfer, m is the mass of the substance,. Heat transfer and temperature change.
From www.youtube.com
Thermochemical Equations and Using the energy term (heat of reaction Temperature Change Energy Equation Interconvert temperatures between celsius, kelvin, and fahrenheit scales A practical approximation for the relationship between heat transfer and temperature change is: Explain that temperature is a measure of internal kinetic energy; Change in thermal energy = mass × specific heat. Q = mcδt, where q is the symbol for heat transfer, m is the mass of the substance,. The quantitative. Temperature Change Energy Equation.
From www.grc.nasa.gov
Specific Heats Temperature Change Energy Equation The change in thermal energy due to temperature changes is calculated using this equation: Nevertheless, heat and work can produce. Heat transfer and temperature change. A practical approximation for the relationship between heat transfer and temperature change is: \[q = mc\delta t,\] where \(q\) is. Multiply the change in temperature with the mass of the sample. Interconvert temperatures between celsius,. Temperature Change Energy Equation.
From www.youtube.com
Calculating thermal energy changes Q=mcdT YouTube Temperature Change Energy Equation Subtract the final and initial temperature to get the change in temperature (δt). Multiply the change in temperature with the mass of the sample. Explain that temperature is a measure of internal kinetic energy; A practical approximation for the relationship between heat transfer and temperature change is: The change in thermal energy due to temperature changes is calculated using this. Temperature Change Energy Equation.
From jackwestin.com
Phase Diagram Pressure And Temperature Energy Changes In Chemical Temperature Change Energy Equation Subtract the final and initial temperature to get the change in temperature (δt). Change in thermal energy = mass × specific heat. Multiply the change in temperature with the mass of the sample. Heat transfer and temperature change. A practical approximation for the relationship between heat transfer and temperature change is: Q = mcδt, where q is the symbol for. Temperature Change Energy Equation.
From www.youtube.com
Example Using specific heat to calculate ideal gas internal energy Temperature Change Energy Equation Nevertheless, heat and work can produce. Change in thermal energy = mass × specific heat. A practical approximation for the relationship between heat transfer and temperature change is: Interconvert temperatures between celsius, kelvin, and fahrenheit scales Heat is driven by temperature differences, while work involves a force exerted through a distance. The quantitative relationship between heat transfer and temperature change. Temperature Change Energy Equation.
From www.youtube.com
Internal Energy, Heat, and Work Thermodynamics, Pressure & Volume Temperature Change Energy Equation Change in thermal energy = mass × specific heat. The quantitative relationship between heat transfer and temperature change contains all three factors: Interconvert temperatures between celsius, kelvin, and fahrenheit scales Subtract the final and initial temperature to get the change in temperature (δt). Heat is driven by temperature differences, while work involves a force exerted through a distance. Nevertheless, heat. Temperature Change Energy Equation.
From www.slideserve.com
PPT ENERGY PowerPoint Presentation, free download ID3232966 Temperature Change Energy Equation \[q = mc\delta t,\] where \(q\) is. Nevertheless, heat and work can produce. Explain that temperature is a measure of internal kinetic energy; Interconvert temperatures between celsius, kelvin, and fahrenheit scales Change in thermal energy = mass × specific heat. Heat transfer and temperature change. Subtract the final and initial temperature to get the change in temperature (δt). A practical. Temperature Change Energy Equation.
From sciencetallis.weebly.com
1. Energy THOMAS TALLIS SCIENCE Temperature Change Energy Equation Heat is driven by temperature differences, while work involves a force exerted through a distance. The quantitative relationship between heat transfer and temperature change contains all three factors: Nevertheless, heat and work can produce. Change in thermal energy = mass × specific heat. Q = mcδt, where q is the symbol for heat transfer, m is the mass of the. Temperature Change Energy Equation.
From studylib.net
Heat Equation Temperature Change Energy Equation Change in thermal energy = mass × specific heat. Subtract the final and initial temperature to get the change in temperature (δt). A practical approximation for the relationship between heat transfer and temperature change is: Nevertheless, heat and work can produce. The quantitative relationship between heat transfer and temperature change contains all three factors: Interconvert temperatures between celsius, kelvin, and. Temperature Change Energy Equation.
From www.grc.nasa.gov
Heat Transfer Temperature Change Energy Equation Multiply the change in temperature with the mass of the sample. Explain that temperature is a measure of internal kinetic energy; The quantitative relationship between heat transfer and temperature change contains all three factors: The change in thermal energy due to temperature changes is calculated using this equation: Q = mcδt, where q is the symbol for heat transfer, m. Temperature Change Energy Equation.
From www.youtube.com
Adiabatic Process Work, Heat & Internal Energy, Gamma Ratio Temperature Change Energy Equation Heat transfer and temperature change. Subtract the final and initial temperature to get the change in temperature (δt). A practical approximation for the relationship between heat transfer and temperature change is: The quantitative relationship between heat transfer and temperature change contains all three factors: \[q = mc\delta t,\] where \(q\) is. Explain that temperature is a measure of internal kinetic. Temperature Change Energy Equation.
From fyohqoqgp.blob.core.windows.net
Calorimeter Formula In Physics at Caroline Graig blog Temperature Change Energy Equation Heat transfer and temperature change. A practical approximation for the relationship between heat transfer and temperature change is: Subtract the final and initial temperature to get the change in temperature (δt). Change in thermal energy = mass × specific heat. \[q = mc\delta t,\] where \(q\) is. Heat is driven by temperature differences, while work involves a force exerted through. Temperature Change Energy Equation.
From study.com
Using Specific Heat Capacity to Find Temperature Change Chemistry Temperature Change Energy Equation Multiply the change in temperature with the mass of the sample. Change in thermal energy = mass × specific heat. Interconvert temperatures between celsius, kelvin, and fahrenheit scales Q = mcδt, where q is the symbol for heat transfer, m is the mass of the substance,. The quantitative relationship between heat transfer and temperature change contains all three factors: Explain. Temperature Change Energy Equation.
From www.teachoo.com
Effect of Temperature to Change State of Matter Teachoo Science Temperature Change Energy Equation Explain that temperature is a measure of internal kinetic energy; Heat transfer and temperature change. A practical approximation for the relationship between heat transfer and temperature change is: The change in thermal energy due to temperature changes is calculated using this equation: \[q = mc\delta t,\] where \(q\) is. The quantitative relationship between heat transfer and temperature change contains all. Temperature Change Energy Equation.
From www.slideserve.com
PPT HEAT EQUATION (in Table T) PowerPoint Presentation, free download Temperature Change Energy Equation A practical approximation for the relationship between heat transfer and temperature change is: Change in thermal energy = mass × specific heat. Heat is driven by temperature differences, while work involves a force exerted through a distance. The change in thermal energy due to temperature changes is calculated using this equation: Heat transfer and temperature change. Multiply the change in. Temperature Change Energy Equation.
From www.youtube.com
Thermodynamics (Physics) Lesson 2 Heat Transfer and Specific Heat.avi Temperature Change Energy Equation \[q = mc\delta t,\] where \(q\) is. Heat is driven by temperature differences, while work involves a force exerted through a distance. Nevertheless, heat and work can produce. A practical approximation for the relationship between heat transfer and temperature change is: Interconvert temperatures between celsius, kelvin, and fahrenheit scales Explain that temperature is a measure of internal kinetic energy; Q. Temperature Change Energy Equation.
From studylib.net
Thermodynamics Formulas Temperature Change Energy Equation Heat is driven by temperature differences, while work involves a force exerted through a distance. Subtract the final and initial temperature to get the change in temperature (δt). The quantitative relationship between heat transfer and temperature change contains all three factors: Heat transfer and temperature change. \[q = mc\delta t,\] where \(q\) is. Explain that temperature is a measure of. Temperature Change Energy Equation.
From www.slideserve.com
PPT Energy, Power and Climate Change PowerPoint Presentation, free Temperature Change Energy Equation A practical approximation for the relationship between heat transfer and temperature change is: Explain that temperature is a measure of internal kinetic energy; Q = mcδt, where q is the symbol for heat transfer, m is the mass of the substance,. Nevertheless, heat and work can produce. Interconvert temperatures between celsius, kelvin, and fahrenheit scales The change in thermal energy. Temperature Change Energy Equation.
From www.youtube.com
First Law of Thermodynamics, Basic Introduction Internal Energy, Heat Temperature Change Energy Equation Multiply the change in temperature with the mass of the sample. A practical approximation for the relationship between heat transfer and temperature change is: The quantitative relationship between heat transfer and temperature change contains all three factors: Explain that temperature is a measure of internal kinetic energy; Subtract the final and initial temperature to get the change in temperature (δt).. Temperature Change Energy Equation.
From www.engineeringstream.com
LAW OF THERMODYNAMICS Engineering Stream Temperature Change Energy Equation Heat is driven by temperature differences, while work involves a force exerted through a distance. The change in thermal energy due to temperature changes is calculated using this equation: Interconvert temperatures between celsius, kelvin, and fahrenheit scales Explain that temperature is a measure of internal kinetic energy; Subtract the final and initial temperature to get the change in temperature (δt).. Temperature Change Energy Equation.
From www.youtube.com
Specific Heat Capacity Solving for Change in Temperature YouTube Temperature Change Energy Equation Heat is driven by temperature differences, while work involves a force exerted through a distance. The change in thermal energy due to temperature changes is calculated using this equation: Interconvert temperatures between celsius, kelvin, and fahrenheit scales Explain that temperature is a measure of internal kinetic energy; \[q = mc\delta t,\] where \(q\) is. Q = mcδt, where q is. Temperature Change Energy Equation.
From www.grc.nasa.gov
enthalpy Temperature Change Energy Equation The quantitative relationship between heat transfer and temperature change contains all three factors: Interconvert temperatures between celsius, kelvin, and fahrenheit scales A practical approximation for the relationship between heat transfer and temperature change is: Heat transfer and temperature change. Multiply the change in temperature with the mass of the sample. \[q = mc\delta t,\] where \(q\) is. Q = mcδt,. Temperature Change Energy Equation.
From www.youtube.com
Specific Heat Equation Stated Clearly YouTube Temperature Change Energy Equation A practical approximation for the relationship between heat transfer and temperature change is: The change in thermal energy due to temperature changes is calculated using this equation: Multiply the change in temperature with the mass of the sample. \[q = mc\delta t,\] where \(q\) is. Subtract the final and initial temperature to get the change in temperature (δt). Nevertheless, heat. Temperature Change Energy Equation.
From studentcomfort26.gitlab.io
Ideal Steady State Energy Balance Equation Physics Provincial Exam Temperature Change Energy Equation Subtract the final and initial temperature to get the change in temperature (δt). Heat transfer and temperature change. Nevertheless, heat and work can produce. The quantitative relationship between heat transfer and temperature change contains all three factors: The change in thermal energy due to temperature changes is calculated using this equation: Interconvert temperatures between celsius, kelvin, and fahrenheit scales \[q. Temperature Change Energy Equation.
From www.youtube.com
Mechanical Engineering Thermodynamics Lec 10, pt 1 of 2 Entropy Temperature Change Energy Equation Subtract the final and initial temperature to get the change in temperature (δt). Interconvert temperatures between celsius, kelvin, and fahrenheit scales Nevertheless, heat and work can produce. \[q = mc\delta t,\] where \(q\) is. Q = mcδt, where q is the symbol for heat transfer, m is the mass of the substance,. The change in thermal energy due to temperature. Temperature Change Energy Equation.
From www.eng-tips.com
About the energy equation for temperature in CFD Heat Transfer Temperature Change Energy Equation Heat transfer and temperature change. Nevertheless, heat and work can produce. Subtract the final and initial temperature to get the change in temperature (δt). The quantitative relationship between heat transfer and temperature change contains all three factors: The change in thermal energy due to temperature changes is calculated using this equation: Q = mcδt, where q is the symbol for. Temperature Change Energy Equation.
From www.youtube.com
Change in Internal Energy Using Specific Heat in 3 Minutes! YouTube Temperature Change Energy Equation Q = mcδt, where q is the symbol for heat transfer, m is the mass of the substance,. Nevertheless, heat and work can produce. Interconvert temperatures between celsius, kelvin, and fahrenheit scales Explain that temperature is a measure of internal kinetic energy; Heat is driven by temperature differences, while work involves a force exerted through a distance. Heat transfer and. Temperature Change Energy Equation.
From www.toppr.com
Specific Heat Formula Definition, Equations, Examples Temperature Change Energy Equation The change in thermal energy due to temperature changes is calculated using this equation: The quantitative relationship between heat transfer and temperature change contains all three factors: Interconvert temperatures between celsius, kelvin, and fahrenheit scales Heat transfer and temperature change. Subtract the final and initial temperature to get the change in temperature (δt). Multiply the change in temperature with the. Temperature Change Energy Equation.
From www.tessshebaylo.com
Equation For Heat Energy From Temperature Change Tessshebaylo Temperature Change Energy Equation Q = mcδt, where q is the symbol for heat transfer, m is the mass of the substance,. A practical approximation for the relationship between heat transfer and temperature change is: Change in thermal energy = mass × specific heat. Multiply the change in temperature with the mass of the sample. \[q = mc\delta t,\] where \(q\) is. Explain that. Temperature Change Energy Equation.
From www.youtube.com
The First Law of Thermodynamics Internal Energy, Heat, and Work YouTube Temperature Change Energy Equation Subtract the final and initial temperature to get the change in temperature (δt). The quantitative relationship between heat transfer and temperature change contains all three factors: Heat transfer and temperature change. Explain that temperature is a measure of internal kinetic energy; Multiply the change in temperature with the mass of the sample. Nevertheless, heat and work can produce. \[q =. Temperature Change Energy Equation.
From www.nagwa.com
Question Video Calculating the Energy Needed to Heat a Mass by a Known Temperature Change Energy Equation Multiply the change in temperature with the mass of the sample. Subtract the final and initial temperature to get the change in temperature (δt). The change in thermal energy due to temperature changes is calculated using this equation: Heat is driven by temperature differences, while work involves a force exerted through a distance. \[q = mc\delta t,\] where \(q\) is.. Temperature Change Energy Equation.
From www.grc.nasa.gov
Conservation of Energy Temperature Change Energy Equation Interconvert temperatures between celsius, kelvin, and fahrenheit scales Heat is driven by temperature differences, while work involves a force exerted through a distance. The quantitative relationship between heat transfer and temperature change contains all three factors: Subtract the final and initial temperature to get the change in temperature (δt). Explain that temperature is a measure of internal kinetic energy; Q. Temperature Change Energy Equation.
From www.tessshebaylo.com
Heat Loss Equation Chemistry Tessshebaylo Temperature Change Energy Equation Subtract the final and initial temperature to get the change in temperature (δt). \[q = mc\delta t,\] where \(q\) is. Nevertheless, heat and work can produce. Change in thermal energy = mass × specific heat. The quantitative relationship between heat transfer and temperature change contains all three factors: The change in thermal energy due to temperature changes is calculated using. Temperature Change Energy Equation.
From www.youtube.com
Calculate Gibbs Free Energy Change for a Reaction at Elevated Temperature Change Energy Equation The quantitative relationship between heat transfer and temperature change contains all three factors: A practical approximation for the relationship between heat transfer and temperature change is: Nevertheless, heat and work can produce. Q = mcδt, where q is the symbol for heat transfer, m is the mass of the substance,. Interconvert temperatures between celsius, kelvin, and fahrenheit scales Heat transfer. Temperature Change Energy Equation.
From www.adda247.com
Energy formula in Physics & Equation for Class 10, 11 and 12 Temperature Change Energy Equation Change in thermal energy = mass × specific heat. Subtract the final and initial temperature to get the change in temperature (δt). The change in thermal energy due to temperature changes is calculated using this equation: Multiply the change in temperature with the mass of the sample. Explain that temperature is a measure of internal kinetic energy; Q = mcδt,. Temperature Change Energy Equation.