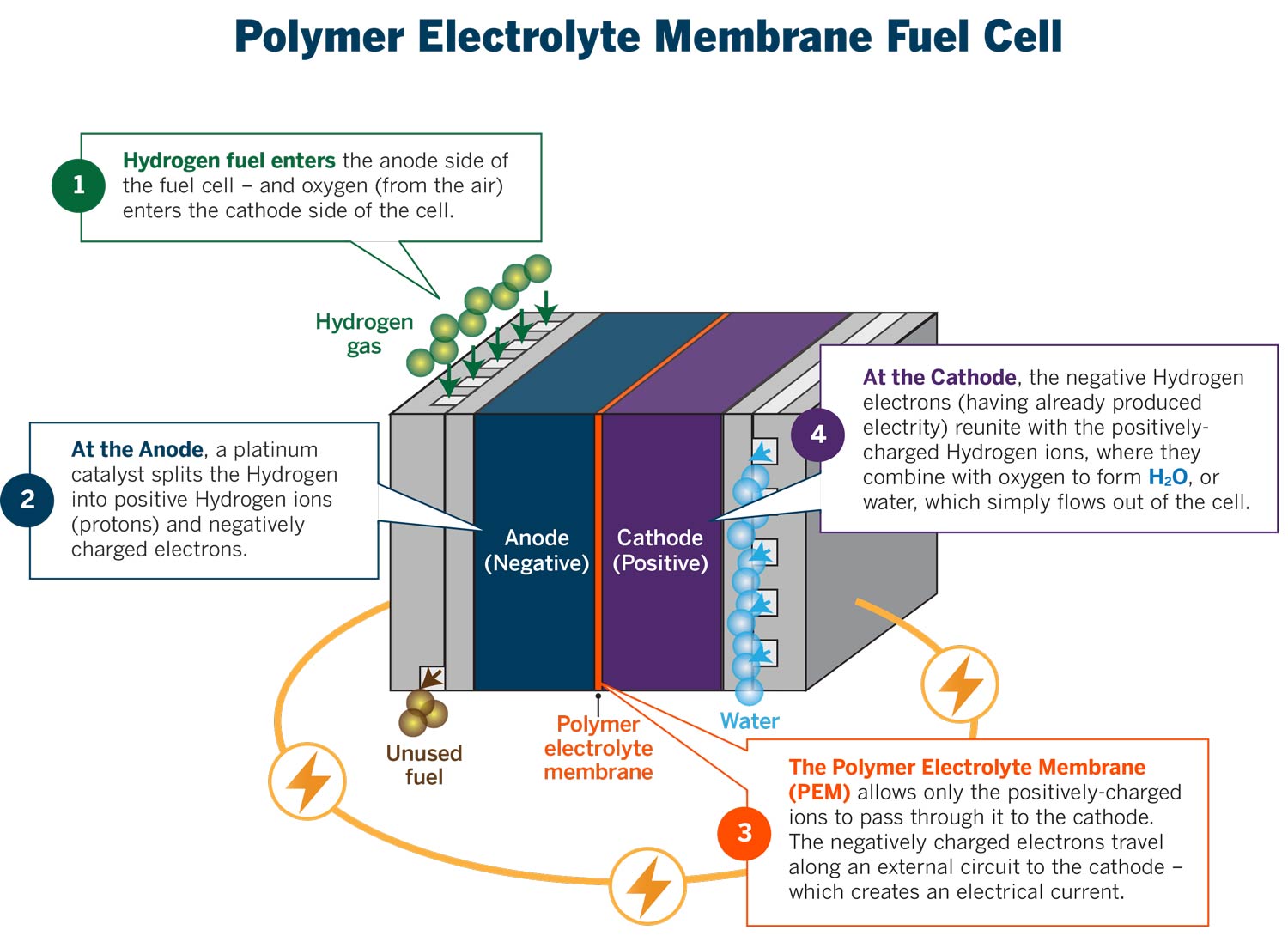
Standard Hydrogen Electrode Fuel Cell . The standard hydrogen electrode the standard hydrogen electrode looks like this: Graphical representation of the onset potentials of the her/hor and oer/orr measured using a. It is also called standard reduction potential. It can produce electric power by inducing both a. Examples of using the standard hydrogen electrode to determine The structure of the standard hydrogen electrode is described. Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. Electrode potential is a measure of reducing power of any element. They consist of an electrolyte medium sandwiched between two electrodes (fig. Fuel cells are electrochemical devices that directly convert chemical energy to electrical energy. Fuel cell is a system of electric power generation, which utilizes electrochemical reactions. The standard hydrogen electrode is constructed so that h 2 gas flows over an inert electrode made of platinum, and can interact. As the hydrogen gas flows over the.
from www.sundyne.com
Examples of using the standard hydrogen electrode to determine They consist of an electrolyte medium sandwiched between two electrodes (fig. It is also called standard reduction potential. The structure of the standard hydrogen electrode is described. The standard hydrogen electrode the standard hydrogen electrode looks like this: It can produce electric power by inducing both a. Graphical representation of the onset potentials of the her/hor and oer/orr measured using a. Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. As the hydrogen gas flows over the. Fuel cells are electrochemical devices that directly convert chemical energy to electrical energy.
Hydrogen Fuel Cells How Do They Work? Sundyne
Standard Hydrogen Electrode Fuel Cell They consist of an electrolyte medium sandwiched between two electrodes (fig. Examples of using the standard hydrogen electrode to determine Graphical representation of the onset potentials of the her/hor and oer/orr measured using a. The structure of the standard hydrogen electrode is described. It is also called standard reduction potential. The standard hydrogen electrode the standard hydrogen electrode looks like this: Fuel cells are electrochemical devices that directly convert chemical energy to electrical energy. It can produce electric power by inducing both a. They consist of an electrolyte medium sandwiched between two electrodes (fig. Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. Electrode potential is a measure of reducing power of any element. As the hydrogen gas flows over the. The standard hydrogen electrode is constructed so that h 2 gas flows over an inert electrode made of platinum, and can interact. Fuel cell is a system of electric power generation, which utilizes electrochemical reactions.
From byjus.com
What is standard hydrogen electrode? Standard Hydrogen Electrode Fuel Cell Electrode potential is a measure of reducing power of any element. It is also called standard reduction potential. As the hydrogen gas flows over the. Fuel cell is a system of electric power generation, which utilizes electrochemical reactions. Graphical representation of the onset potentials of the her/hor and oer/orr measured using a. They consist of an electrolyte medium sandwiched between. Standard Hydrogen Electrode Fuel Cell.
From mmerevise.co.uk
Electrochemical Cells MME Standard Hydrogen Electrode Fuel Cell The standard hydrogen electrode the standard hydrogen electrode looks like this: Fuel cell is a system of electric power generation, which utilizes electrochemical reactions. Electrode potential is a measure of reducing power of any element. Fuel cells are electrochemical devices that directly convert chemical energy to electrical energy. It can produce electric power by inducing both a. Graphical representation of. Standard Hydrogen Electrode Fuel Cell.
From stock.adobe.com
A Standard Hydrogen Electrode (SHE) is an electrode that scientists use Standard Hydrogen Electrode Fuel Cell The standard hydrogen electrode is constructed so that h 2 gas flows over an inert electrode made of platinum, and can interact. Graphical representation of the onset potentials of the her/hor and oer/orr measured using a. Electrode potential is a measure of reducing power of any element. The standard hydrogen electrode the standard hydrogen electrode looks like this: The structure. Standard Hydrogen Electrode Fuel Cell.
From www.slideserve.com
PPT Chapter 21 Electrochemistry PowerPoint Presentation, free Standard Hydrogen Electrode Fuel Cell Graphical representation of the onset potentials of the her/hor and oer/orr measured using a. Examples of using the standard hydrogen electrode to determine The structure of the standard hydrogen electrode is described. Fuel cells are electrochemical devices that directly convert chemical energy to electrical energy. As the hydrogen gas flows over the. Standard hydrogen electrode is used as a reference. Standard Hydrogen Electrode Fuel Cell.
From www.slideserve.com
PPT Standard hydrogen electrode PowerPoint Presentation, free Standard Hydrogen Electrode Fuel Cell The standard hydrogen electrode is constructed so that h 2 gas flows over an inert electrode made of platinum, and can interact. Examples of using the standard hydrogen electrode to determine Graphical representation of the onset potentials of the her/hor and oer/orr measured using a. Fuel cells are electrochemical devices that directly convert chemical energy to electrical energy. It is. Standard Hydrogen Electrode Fuel Cell.
From saylordotorg.github.io
Standard Potentials Standard Hydrogen Electrode Fuel Cell Fuel cell is a system of electric power generation, which utilizes electrochemical reactions. It is also called standard reduction potential. Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. The structure of the standard hydrogen electrode is described. Fuel cells are electrochemical devices that directly convert chemical energy to electrical. Standard Hydrogen Electrode Fuel Cell.
From nadeesharangikacarguy.blogspot.com
The Car Guy Hydrogen fuel cell technology for cars Standard Hydrogen Electrode Fuel Cell It is also called standard reduction potential. Examples of using the standard hydrogen electrode to determine Fuel cells are electrochemical devices that directly convert chemical energy to electrical energy. It can produce electric power by inducing both a. Graphical representation of the onset potentials of the her/hor and oer/orr measured using a. Fuel cell is a system of electric power. Standard Hydrogen Electrode Fuel Cell.
From monomole.com
Standard hydrogen electrode Mono Mole Standard Hydrogen Electrode Fuel Cell Graphical representation of the onset potentials of the her/hor and oer/orr measured using a. Fuel cell is a system of electric power generation, which utilizes electrochemical reactions. Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. It is also called standard reduction potential. It can produce electric power by inducing. Standard Hydrogen Electrode Fuel Cell.
From large.stanford.edu
Hydrogen Fuel Cells Standard Hydrogen Electrode Fuel Cell It is also called standard reduction potential. The standard hydrogen electrode the standard hydrogen electrode looks like this: They consist of an electrolyte medium sandwiched between two electrodes (fig. Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. Examples of using the standard hydrogen electrode to determine The standard hydrogen. Standard Hydrogen Electrode Fuel Cell.
From www.lapsedphysicist.org
Hydrogen and Fuel Cells Important Parts of Our Energy Future Standard Hydrogen Electrode Fuel Cell The standard hydrogen electrode is constructed so that h 2 gas flows over an inert electrode made of platinum, and can interact. It can produce electric power by inducing both a. As the hydrogen gas flows over the. Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. They consist of. Standard Hydrogen Electrode Fuel Cell.
From pubs.acs.org
Standard and Reversible Hydrogen Electrodes Theory, Design, Operation Standard Hydrogen Electrode Fuel Cell Fuel cells are electrochemical devices that directly convert chemical energy to electrical energy. Electrode potential is a measure of reducing power of any element. Examples of using the standard hydrogen electrode to determine Fuel cell is a system of electric power generation, which utilizes electrochemical reactions. As the hydrogen gas flows over the. The standard hydrogen electrode the standard hydrogen. Standard Hydrogen Electrode Fuel Cell.
From scienceinfo.com
What is Standard Hydrogen Electrode? Standard Hydrogen Electrode Fuel Cell It is also called standard reduction potential. Electrode potential is a measure of reducing power of any element. Examples of using the standard hydrogen electrode to determine The standard hydrogen electrode the standard hydrogen electrode looks like this: The structure of the standard hydrogen electrode is described. Graphical representation of the onset potentials of the her/hor and oer/orr measured using. Standard Hydrogen Electrode Fuel Cell.
From www.setra.com
What is a Hydrogen Fuel Cell and How Does it Work? Standard Hydrogen Electrode Fuel Cell Electrode potential is a measure of reducing power of any element. The structure of the standard hydrogen electrode is described. Examples of using the standard hydrogen electrode to determine Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. The standard hydrogen electrode is constructed so that h 2 gas flows. Standard Hydrogen Electrode Fuel Cell.
From www.sundyne.com
Hydrogen Fuel Cells How Do They Work? Sundyne Standard Hydrogen Electrode Fuel Cell Fuel cells are electrochemical devices that directly convert chemical energy to electrical energy. They consist of an electrolyte medium sandwiched between two electrodes (fig. As the hydrogen gas flows over the. Examples of using the standard hydrogen electrode to determine It is also called standard reduction potential. Electrode potential is a measure of reducing power of any element. The standard. Standard Hydrogen Electrode Fuel Cell.
From saylordotorg.github.io
Standard Potentials Standard Hydrogen Electrode Fuel Cell Graphical representation of the onset potentials of the her/hor and oer/orr measured using a. Electrode potential is a measure of reducing power of any element. It is also called standard reduction potential. Fuel cells are electrochemical devices that directly convert chemical energy to electrical energy. Fuel cell is a system of electric power generation, which utilizes electrochemical reactions. The standard. Standard Hydrogen Electrode Fuel Cell.
From www.chfca.ca
About Fuel Cells CHFCA Standard Hydrogen Electrode Fuel Cell Examples of using the standard hydrogen electrode to determine It can produce electric power by inducing both a. Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. They consist of an electrolyte medium sandwiched between two electrodes (fig. As the hydrogen gas flows over the. The standard hydrogen electrode is. Standard Hydrogen Electrode Fuel Cell.
From www.thoughtco.com
Standard Hydrogen Electrode Chemistry Definition Standard Hydrogen Electrode Fuel Cell It is also called standard reduction potential. Fuel cells are electrochemical devices that directly convert chemical energy to electrical energy. Electrode potential is a measure of reducing power of any element. Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. As the hydrogen gas flows over the. The standard hydrogen. Standard Hydrogen Electrode Fuel Cell.
From www.savemyexams.com
The Hydrogen Electrode (HL) HL IB Chemistry Revision Notes 2025 Standard Hydrogen Electrode Fuel Cell Examples of using the standard hydrogen electrode to determine The standard hydrogen electrode the standard hydrogen electrode looks like this: Fuel cells are electrochemical devices that directly convert chemical energy to electrical energy. The structure of the standard hydrogen electrode is described. Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half. Standard Hydrogen Electrode Fuel Cell.
From www.britannica.com
Fuel cell Proton Exchange, Alkaline, Polymer Britannica Standard Hydrogen Electrode Fuel Cell Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. Electrode potential is a measure of reducing power of any element. It can produce electric power by inducing both a. Fuel cells are electrochemical devices that directly convert chemical energy to electrical energy. The standard hydrogen electrode the standard hydrogen electrode. Standard Hydrogen Electrode Fuel Cell.
From www.researchgate.net
e Schematic view of a typical hydrogen PEM fuel cell and its components Standard Hydrogen Electrode Fuel Cell Fuel cells are electrochemical devices that directly convert chemical energy to electrical energy. Examples of using the standard hydrogen electrode to determine As the hydrogen gas flows over the. Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. Graphical representation of the onset potentials of the her/hor and oer/orr measured. Standard Hydrogen Electrode Fuel Cell.
From www.slideserve.com
PPT Air Electrode in Hydrogen Fuel Cell PowerPoint Presentation, free Standard Hydrogen Electrode Fuel Cell The standard hydrogen electrode is constructed so that h 2 gas flows over an inert electrode made of platinum, and can interact. The structure of the standard hydrogen electrode is described. They consist of an electrolyte medium sandwiched between two electrodes (fig. It is also called standard reduction potential. Electrode potential is a measure of reducing power of any element.. Standard Hydrogen Electrode Fuel Cell.
From question.pandai.org
Standard Electrode Potential Standard Hydrogen Electrode Fuel Cell Fuel cell is a system of electric power generation, which utilizes electrochemical reactions. As the hydrogen gas flows over the. It can produce electric power by inducing both a. They consist of an electrolyte medium sandwiched between two electrodes (fig. It is also called standard reduction potential. Fuel cells are electrochemical devices that directly convert chemical energy to electrical energy.. Standard Hydrogen Electrode Fuel Cell.
From 640orfree.com
How Does A Hydrogen Fuel Cell Work? A Comprehensive Guide Linquip (2022) Standard Hydrogen Electrode Fuel Cell Examples of using the standard hydrogen electrode to determine They consist of an electrolyte medium sandwiched between two electrodes (fig. Electrode potential is a measure of reducing power of any element. The standard hydrogen electrode the standard hydrogen electrode looks like this: Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half. Standard Hydrogen Electrode Fuel Cell.
From www.change-florida.com
Hydrogen Fuel Cells Standard Hydrogen Electrode Fuel Cell The standard hydrogen electrode the standard hydrogen electrode looks like this: Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. Examples of using the standard hydrogen electrode to determine Fuel cell is a system of electric power generation, which utilizes electrochemical reactions. As the hydrogen gas flows over the. Graphical. Standard Hydrogen Electrode Fuel Cell.
From saylordotorg.github.io
Electrochemistry Standard Hydrogen Electrode Fuel Cell The standard hydrogen electrode is constructed so that h 2 gas flows over an inert electrode made of platinum, and can interact. The standard hydrogen electrode the standard hydrogen electrode looks like this: Fuel cell is a system of electric power generation, which utilizes electrochemical reactions. Graphical representation of the onset potentials of the her/hor and oer/orr measured using a.. Standard Hydrogen Electrode Fuel Cell.
From www.electroniclinic.com
Hydrogen Fuel Cell, Application of Fuel Cells, construction, and Working Standard Hydrogen Electrode Fuel Cell It is also called standard reduction potential. Examples of using the standard hydrogen electrode to determine It can produce electric power by inducing both a. The standard hydrogen electrode is constructed so that h 2 gas flows over an inert electrode made of platinum, and can interact. Fuel cells are electrochemical devices that directly convert chemical energy to electrical energy.. Standard Hydrogen Electrode Fuel Cell.
From www.slideserve.com
PPT Chemistry PowerPoint Presentation, free download ID752723 Standard Hydrogen Electrode Fuel Cell Examples of using the standard hydrogen electrode to determine Electrode potential is a measure of reducing power of any element. Fuel cell is a system of electric power generation, which utilizes electrochemical reactions. It is also called standard reduction potential. They consist of an electrolyte medium sandwiched between two electrodes (fig. The standard hydrogen electrode the standard hydrogen electrode looks. Standard Hydrogen Electrode Fuel Cell.
From stock.adobe.com
Standard hydrogen electrode diagram. Scientific vector illustration Standard Hydrogen Electrode Fuel Cell Graphical representation of the onset potentials of the her/hor and oer/orr measured using a. Electrode potential is a measure of reducing power of any element. It can produce electric power by inducing both a. The structure of the standard hydrogen electrode is described. Fuel cells are electrochemical devices that directly convert chemical energy to electrical energy. The standard hydrogen electrode. Standard Hydrogen Electrode Fuel Cell.
From joilpfrjj.blob.core.windows.net
Polymer Electrolyte Fuel Cells Cathode at Miguel Morrell blog Standard Hydrogen Electrode Fuel Cell The standard hydrogen electrode is constructed so that h 2 gas flows over an inert electrode made of platinum, and can interact. The structure of the standard hydrogen electrode is described. Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. As the hydrogen gas flows over the. Examples of using. Standard Hydrogen Electrode Fuel Cell.
From kladutthv.blob.core.windows.net
Fuel Cell Electrolysis at Howard Rooks blog Standard Hydrogen Electrode Fuel Cell The standard hydrogen electrode is constructed so that h 2 gas flows over an inert electrode made of platinum, and can interact. As the hydrogen gas flows over the. Graphical representation of the onset potentials of the her/hor and oer/orr measured using a. They consist of an electrolyte medium sandwiched between two electrodes (fig. The standard hydrogen electrode the standard. Standard Hydrogen Electrode Fuel Cell.
From www.slideserve.com
PPT Electroanalysis PowerPoint Presentation, free download ID6733376 Standard Hydrogen Electrode Fuel Cell Electrode potential is a measure of reducing power of any element. Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. It is also called standard reduction potential. Fuel cells are electrochemical devices that directly convert chemical energy to electrical energy. Examples of using the standard hydrogen electrode to determine It. Standard Hydrogen Electrode Fuel Cell.
From totalshield.com
Electrolyzer and Hydrogen Fuel Cell Safety TotalShield Standard Hydrogen Electrode Fuel Cell The standard hydrogen electrode the standard hydrogen electrode looks like this: It can produce electric power by inducing both a. Electrode potential is a measure of reducing power of any element. As the hydrogen gas flows over the. Graphical representation of the onset potentials of the her/hor and oer/orr measured using a. Examples of using the standard hydrogen electrode to. Standard Hydrogen Electrode Fuel Cell.
From byjus.com
Standard Hydrogen Electrode Definition, Construction, and Labelled Standard Hydrogen Electrode Fuel Cell It can produce electric power by inducing both a. The standard hydrogen electrode the standard hydrogen electrode looks like this: The standard hydrogen electrode is constructed so that h 2 gas flows over an inert electrode made of platinum, and can interact. Examples of using the standard hydrogen electrode to determine Graphical representation of the onset potentials of the her/hor. Standard Hydrogen Electrode Fuel Cell.
From www.slideserve.com
PPT Chapter 17 Electrochemistry PowerPoint Presentation, free Standard Hydrogen Electrode Fuel Cell Fuel cell is a system of electric power generation, which utilizes electrochemical reactions. Examples of using the standard hydrogen electrode to determine Electrode potential is a measure of reducing power of any element. It is also called standard reduction potential. They consist of an electrolyte medium sandwiched between two electrodes (fig. Graphical representation of the onset potentials of the her/hor. Standard Hydrogen Electrode Fuel Cell.
From www.youtube.com
19.1 Standard hydrogen electrode (HL) YouTube Standard Hydrogen Electrode Fuel Cell It can produce electric power by inducing both a. As the hydrogen gas flows over the. Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. Fuel cell is a system of electric power generation, which utilizes electrochemical reactions. The standard hydrogen electrode is constructed so that h 2 gas flows. Standard Hydrogen Electrode Fuel Cell.