Device Labeling Guidance #G91-1 . Fda proposes naming conventions that would include elemental or chemical names (rather than abbreviations), trade names, animal species and tissue type, plant or bacterial source. Reviewers concerning their review of labeling in device marketing submissionsr especially premarket approval applications (phas). Accessory device that is in conflict with the parent device. Labeling guidance is provided below: Formalizes guidance to ode reviewers concerning their review of labeling in device marketing submissionsr especially premarket approval. The document provides guidance for fda reviewers on evaluating device labeling in marketing submissions, especially for premarket approval applications. Labeling blue book memorandum #g91. Food and drug administration (fda) issue date: It aims to ensure labeling. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Based on the definitions for “contraindications” and “warnings” from the. Copies of labeling for the predicate device(s) is recommended.
from clin-r.com
Accessory device that is in conflict with the parent device. Formalizes guidance to ode reviewers concerning their review of labeling in device marketing submissionsr especially premarket approval. The document provides guidance for fda reviewers on evaluating device labeling in marketing submissions, especially for premarket approval applications. Labeling guidance is provided below: Copies of labeling for the predicate device(s) is recommended. Labeling blue book memorandum #g91. Fda proposes naming conventions that would include elemental or chemical names (rather than abbreviations), trade names, animal species and tissue type, plant or bacterial source. Food and drug administration (fda) issue date: Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Reviewers concerning their review of labeling in device marketing submissionsr especially premarket approval applications (phas).
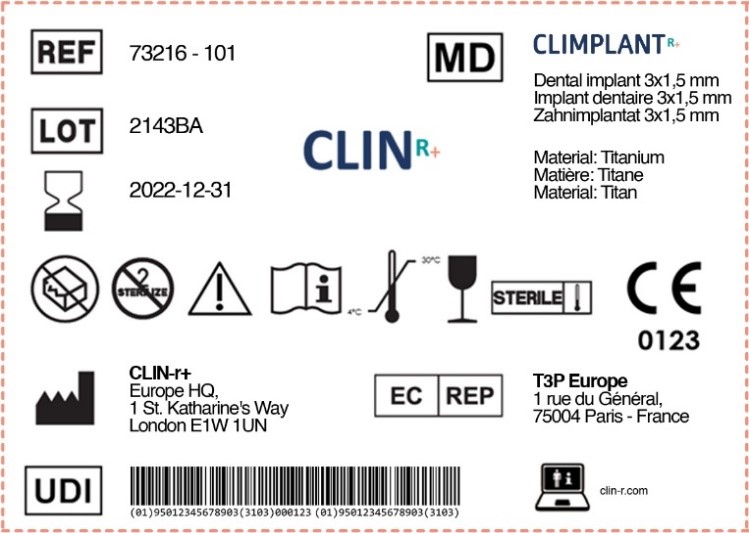
Labels for Medical Devices Clin R
Device Labeling Guidance #G91-1 Labeling blue book memorandum #g91. Formalizes guidance to ode reviewers concerning their review of labeling in device marketing submissionsr especially premarket approval. It aims to ensure labeling. Copies of labeling for the predicate device(s) is recommended. Fda proposes naming conventions that would include elemental or chemical names (rather than abbreviations), trade names, animal species and tissue type, plant or bacterial source. Accessory device that is in conflict with the parent device. The document provides guidance for fda reviewers on evaluating device labeling in marketing submissions, especially for premarket approval applications. Labeling guidance is provided below: Based on the definitions for “contraindications” and “warnings” from the. Labeling blue book memorandum #g91. Reviewers concerning their review of labeling in device marketing submissionsr especially premarket approval applications (phas). Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Food and drug administration (fda) issue date:
From clin-r.com
Labels for Medical Devices Clin R Device Labeling Guidance #G91-1 Accessory device that is in conflict with the parent device. Labeling guidance is provided below: It aims to ensure labeling. Fda proposes naming conventions that would include elemental or chemical names (rather than abbreviations), trade names, animal species and tissue type, plant or bacterial source. The document provides guidance for fda reviewers on evaluating device labeling in marketing submissions, especially. Device Labeling Guidance #G91-1.
From mavink.com
Medical Device Labeling Symbols Device Labeling Guidance #G91-1 Accessory device that is in conflict with the parent device. Reviewers concerning their review of labeling in device marketing submissionsr especially premarket approval applications (phas). Formalizes guidance to ode reviewers concerning their review of labeling in device marketing submissionsr especially premarket approval. Copies of labeling for the predicate device(s) is recommended. Based on the definitions for “contraindications” and “warnings” from. Device Labeling Guidance #G91-1.
From www.slideserve.com
PPT Medical Device Labeling PowerPoint Presentation, free download Device Labeling Guidance #G91-1 Food and drug administration (fda) issue date: Fda proposes naming conventions that would include elemental or chemical names (rather than abbreviations), trade names, animal species and tissue type, plant or bacterial source. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Based on the definitions for “contraindications”. Device Labeling Guidance #G91-1.
From instrktiv.com
IFU for Medical Devices, a Definitive Guide (EU & US) Device Labeling Guidance #G91-1 Food and drug administration (fda) issue date: Accessory device that is in conflict with the parent device. The document provides guidance for fda reviewers on evaluating device labeling in marketing submissions, especially for premarket approval applications. Copies of labeling for the predicate device(s) is recommended. Labeling regulations pertaining to medical devices are found in the following parts of title 21. Device Labeling Guidance #G91-1.
From www.regdesk.co
FDA Guidance on General Device Labeling RegDesk Device Labeling Guidance #G91-1 Based on the definitions for “contraindications” and “warnings” from the. Fda proposes naming conventions that would include elemental or chemical names (rather than abbreviations), trade names, animal species and tissue type, plant or bacterial source. Labeling blue book memorandum #g91. It aims to ensure labeling. The document provides guidance for fda reviewers on evaluating device labeling in marketing submissions, especially. Device Labeling Guidance #G91-1.
From www.productcompliancemanager.com
Commission Published Guidance on Medical Devices Labeling Requirements Device Labeling Guidance #G91-1 Food and drug administration (fda) issue date: Accessory device that is in conflict with the parent device. Reviewers concerning their review of labeling in device marketing submissionsr especially premarket approval applications (phas). Fda proposes naming conventions that would include elemental or chemical names (rather than abbreviations), trade names, animal species and tissue type, plant or bacterial source. It aims to. Device Labeling Guidance #G91-1.
From gbu-taganskij.ru
EU MDR 2017/745 Medical Device Labeling Compliance, 48 OFF Device Labeling Guidance #G91-1 Food and drug administration (fda) issue date: Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Labeling guidance is provided below: Accessory device that is in conflict with the parent device. Labeling blue book memorandum #g91. Copies of labeling for the predicate device(s) is recommended. The document. Device Labeling Guidance #G91-1.
From vivafda.com
FDA Medical Device Labeling Requirements Viva FDA U.S. FDA Device Labeling Guidance #G91-1 Based on the definitions for “contraindications” and “warnings” from the. Fda proposes naming conventions that would include elemental or chemical names (rather than abbreviations), trade names, animal species and tissue type, plant or bacterial source. Labeling blue book memorandum #g91. Food and drug administration (fda) issue date: Formalizes guidance to ode reviewers concerning their review of labeling in device marketing. Device Labeling Guidance #G91-1.
From www.lexology.com
FDA Issues Final Rule on Use of Symbols in Labeling Lexology Device Labeling Guidance #G91-1 Food and drug administration (fda) issue date: Copies of labeling for the predicate device(s) is recommended. Fda proposes naming conventions that would include elemental or chemical names (rather than abbreviations), trade names, animal species and tissue type, plant or bacterial source. The document provides guidance for fda reviewers on evaluating device labeling in marketing submissions, especially for premarket approval applications.. Device Labeling Guidance #G91-1.
From dandelionsandthings.blogspot.com
30 Medical Device Label Symbols Label Design Ideas 2020 Device Labeling Guidance #G91-1 It aims to ensure labeling. Accessory device that is in conflict with the parent device. Reviewers concerning their review of labeling in device marketing submissionsr especially premarket approval applications (phas). Labeling blue book memorandum #g91. Formalizes guidance to ode reviewers concerning their review of labeling in device marketing submissionsr especially premarket approval. Labeling guidance is provided below: Food and drug. Device Labeling Guidance #G91-1.
From vascufirst.com
What is the meaning of symbols on medical devices labels? VascuFirst Device Labeling Guidance #G91-1 Food and drug administration (fda) issue date: Accessory device that is in conflict with the parent device. Copies of labeling for the predicate device(s) is recommended. Reviewers concerning their review of labeling in device marketing submissionsr especially premarket approval applications (phas). The document provides guidance for fda reviewers on evaluating device labeling in marketing submissions, especially for premarket approval applications.. Device Labeling Guidance #G91-1.
From www.vrogue.co
Medical Device Labeling Requirements What You Need To vrogue.co Device Labeling Guidance #G91-1 Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Based on the definitions for “contraindications” and “warnings” from the. Accessory device that is in conflict with the parent device. It aims to ensure labeling. Fda proposes naming conventions that would include elemental or chemical names (rather than. Device Labeling Guidance #G91-1.
From www.pdffiller.com
Fillable Online Device Labeling Guidance G911 (Blue Book Memo) Fax Device Labeling Guidance #G91-1 The document provides guidance for fda reviewers on evaluating device labeling in marketing submissions, especially for premarket approval applications. Labeling blue book memorandum #g91. It aims to ensure labeling. Based on the definitions for “contraindications” and “warnings” from the. Accessory device that is in conflict with the parent device. Formalizes guidance to ode reviewers concerning their review of labeling in. Device Labeling Guidance #G91-1.
From www.regdesk.co
FDA Guidance on Development of Medical Device Labeling RegDesk Device Labeling Guidance #G91-1 Accessory device that is in conflict with the parent device. Reviewers concerning their review of labeling in device marketing submissionsr especially premarket approval applications (phas). Food and drug administration (fda) issue date: Fda proposes naming conventions that would include elemental or chemical names (rather than abbreviations), trade names, animal species and tissue type, plant or bacterial source. Formalizes guidance to. Device Labeling Guidance #G91-1.
From www.scribd.com
Device Labeling Guidance G911 (Blue Book Memo) FDA PDF Federal Device Labeling Guidance #G91-1 Reviewers concerning their review of labeling in device marketing submissionsr especially premarket approval applications (phas). Accessory device that is in conflict with the parent device. Labeling blue book memorandum #g91. Food and drug administration (fda) issue date: Based on the definitions for “contraindications” and “warnings” from the. Fda proposes naming conventions that would include elemental or chemical names (rather than. Device Labeling Guidance #G91-1.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Device Labeling Guidance #G91-1 Copies of labeling for the predicate device(s) is recommended. The document provides guidance for fda reviewers on evaluating device labeling in marketing submissions, especially for premarket approval applications. It aims to ensure labeling. Fda proposes naming conventions that would include elemental or chemical names (rather than abbreviations), trade names, animal species and tissue type, plant or bacterial source. Formalizes guidance. Device Labeling Guidance #G91-1.
From www.regdesk.co
HSA Guidance on UDI System Components and Labeling RegDesk Device Labeling Guidance #G91-1 Food and drug administration (fda) issue date: Based on the definitions for “contraindications” and “warnings” from the. Reviewers concerning their review of labeling in device marketing submissionsr especially premarket approval applications (phas). Labeling blue book memorandum #g91. Labeling guidance is provided below: Accessory device that is in conflict with the parent device. Labeling regulations pertaining to medical devices are found. Device Labeling Guidance #G91-1.
From printingatoms.com
G90 And G91 GCode Commands How To Use Device Labeling Guidance #G91-1 Labeling blue book memorandum #g91. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Food and drug administration (fda) issue date: Formalizes guidance to ode reviewers concerning their review of labeling in device marketing submissionsr especially premarket approval. Accessory device that is in conflict with the parent. Device Labeling Guidance #G91-1.
From www.pinterest.com
G90 and G91 G Codes Cnc software, Cnc programming, Coding Device Labeling Guidance #G91-1 Formalizes guidance to ode reviewers concerning their review of labeling in device marketing submissionsr especially premarket approval. Fda proposes naming conventions that would include elemental or chemical names (rather than abbreviations), trade names, animal species and tissue type, plant or bacterial source. Reviewers concerning their review of labeling in device marketing submissionsr especially premarket approval applications (phas). Accessory device that. Device Labeling Guidance #G91-1.
From www.regdesk.co
HSA Guidance on Labeling for Medical Devices Introduction RegDesk Device Labeling Guidance #G91-1 Based on the definitions for “contraindications” and “warnings” from the. Formalizes guidance to ode reviewers concerning their review of labeling in device marketing submissionsr especially premarket approval. It aims to ensure labeling. Labeling blue book memorandum #g91. Reviewers concerning their review of labeling in device marketing submissionsr especially premarket approval applications (phas). Labeling regulations pertaining to medical devices are found. Device Labeling Guidance #G91-1.
From www.regdesk.co
FDA on General Principles of Labeling for Medical Devices RegDesk Device Labeling Guidance #G91-1 Labeling blue book memorandum #g91. Copies of labeling for the predicate device(s) is recommended. Labeling guidance is provided below: Food and drug administration (fda) issue date: Fda proposes naming conventions that would include elemental or chemical names (rather than abbreviations), trade names, animal species and tissue type, plant or bacterial source. Based on the definitions for “contraindications” and “warnings” from. Device Labeling Guidance #G91-1.
From www.gilero.com
Medical Device Classification Overview of 3 Classes Gilero Device Labeling Guidance #G91-1 It aims to ensure labeling. Copies of labeling for the predicate device(s) is recommended. Labeling blue book memorandum #g91. Reviewers concerning their review of labeling in device marketing submissionsr especially premarket approval applications (phas). Accessory device that is in conflict with the parent device. The document provides guidance for fda reviewers on evaluating device labeling in marketing submissions, especially for. Device Labeling Guidance #G91-1.
From www.regdesk.co
FDA Guidance on General Device Labeling RegDesk Device Labeling Guidance #G91-1 Reviewers concerning their review of labeling in device marketing submissionsr especially premarket approval applications (phas). Food and drug administration (fda) issue date: Labeling blue book memorandum #g91. Copies of labeling for the predicate device(s) is recommended. Labeling guidance is provided below: Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of. Device Labeling Guidance #G91-1.
From mungfali.com
Medical Device Labeling Symbols Device Labeling Guidance #G91-1 Based on the definitions for “contraindications” and “warnings” from the. Formalizes guidance to ode reviewers concerning their review of labeling in device marketing submissionsr especially premarket approval. Fda proposes naming conventions that would include elemental or chemical names (rather than abbreviations), trade names, animal species and tissue type, plant or bacterial source. Reviewers concerning their review of labeling in device. Device Labeling Guidance #G91-1.
From www.gmdesigndevelopment.com
MEDICAL DEVICE SYMBOLS Gm Design Development UK Device Labeling Guidance #G91-1 Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Reviewers concerning their review of labeling in device marketing submissionsr especially premarket approval applications (phas). Copies of labeling for the predicate device(s) is recommended. Fda proposes naming conventions that would include elemental or chemical names (rather than abbreviations),. Device Labeling Guidance #G91-1.
From www.machinistguides.com
Quick Guide to the G91 CNC G Code [Tips and Tricks] Device Labeling Guidance #G91-1 Copies of labeling for the predicate device(s) is recommended. Formalizes guidance to ode reviewers concerning their review of labeling in device marketing submissionsr especially premarket approval. The document provides guidance for fda reviewers on evaluating device labeling in marketing submissions, especially for premarket approval applications. Food and drug administration (fda) issue date: Accessory device that is in conflict with the. Device Labeling Guidance #G91-1.
From www.vrogue.co
Nicelabel Blog Medical Device Labeling Compliance Cha vrogue.co Device Labeling Guidance #G91-1 Food and drug administration (fda) issue date: Based on the definitions for “contraindications” and “warnings” from the. It aims to ensure labeling. Copies of labeling for the predicate device(s) is recommended. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Accessory device that is in conflict with. Device Labeling Guidance #G91-1.
From www.regdesk.co
EFDA Guidance on Medical Device Labeling Special Requirements RegDesk Device Labeling Guidance #G91-1 Food and drug administration (fda) issue date: Accessory device that is in conflict with the parent device. Reviewers concerning their review of labeling in device marketing submissionsr especially premarket approval applications (phas). It aims to ensure labeling. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). The. Device Labeling Guidance #G91-1.
From www.bluproducts.com
G91 MAX Optimal Balance Device Labeling Guidance #G91-1 Formalizes guidance to ode reviewers concerning their review of labeling in device marketing submissionsr especially premarket approval. Fda proposes naming conventions that would include elemental or chemical names (rather than abbreviations), trade names, animal species and tissue type, plant or bacterial source. Copies of labeling for the predicate device(s) is recommended. Based on the definitions for “contraindications” and “warnings” from. Device Labeling Guidance #G91-1.
From www.regdesk.co
FDA Guidance on General Device Labeling RegDesk Device Labeling Guidance #G91-1 Fda proposes naming conventions that would include elemental or chemical names (rather than abbreviations), trade names, animal species and tissue type, plant or bacterial source. The document provides guidance for fda reviewers on evaluating device labeling in marketing submissions, especially for premarket approval applications. Based on the definitions for “contraindications” and “warnings” from the. Labeling regulations pertaining to medical devices. Device Labeling Guidance #G91-1.
From www.meddeviceonline.com
Medical Device Labeling New ISO 152231 FDA Guidance UDI Device Labeling Guidance #G91-1 It aims to ensure labeling. Based on the definitions for “contraindications” and “warnings” from the. Labeling guidance is provided below: Food and drug administration (fda) issue date: Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Formalizes guidance to ode reviewers concerning their review of labeling in. Device Labeling Guidance #G91-1.
From www.pdffiller.com
Fillable Online Device Labeling Guidance G911 (Blue Book Memo)FDA Fax Device Labeling Guidance #G91-1 Copies of labeling for the predicate device(s) is recommended. Food and drug administration (fda) issue date: Fda proposes naming conventions that would include elemental or chemical names (rather than abbreviations), trade names, animal species and tissue type, plant or bacterial source. Based on the definitions for “contraindications” and “warnings” from the. Formalizes guidance to ode reviewers concerning their review of. Device Labeling Guidance #G91-1.
From www.youtube.com
FDA Requirements for Device Labeling YouTube Device Labeling Guidance #G91-1 Accessory device that is in conflict with the parent device. Formalizes guidance to ode reviewers concerning their review of labeling in device marketing submissionsr especially premarket approval. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Labeling guidance is provided below: Reviewers concerning their review of labeling. Device Labeling Guidance #G91-1.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Device Labeling Guidance #G91-1 Reviewers concerning their review of labeling in device marketing submissionsr especially premarket approval applications (phas). The document provides guidance for fda reviewers on evaluating device labeling in marketing submissions, especially for premarket approval applications. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Copies of labeling for. Device Labeling Guidance #G91-1.
From www.scribd.com
Medical Device Labeling New ISO 152231 & FDA Guidance UDI Device Labeling Guidance #G91-1 Accessory device that is in conflict with the parent device. Food and drug administration (fda) issue date: Copies of labeling for the predicate device(s) is recommended. Labeling guidance is provided below: Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Based on the definitions for “contraindications” and. Device Labeling Guidance #G91-1.