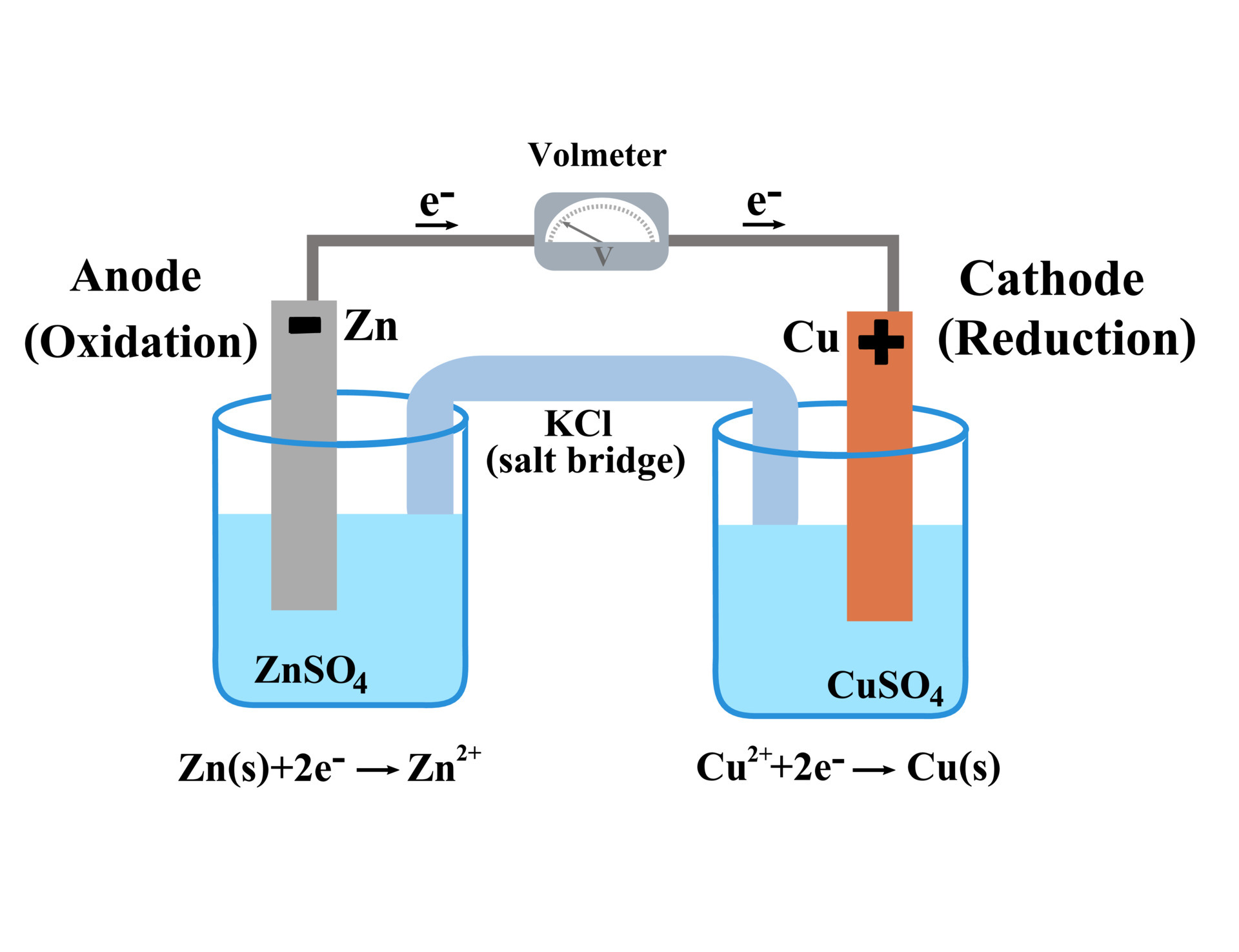
Electrode Chemical Cell . By convention, the electrode written to the left of the salt bridge in this cell notation is always taken to be the anode, and the associated half. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction is called an electrochemical cell. Galvanic cells and electrolytic cells. This kind of cell includes the galvanic, or voltaic, cell, named after. There are two types of electrochemical cells: An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. When you connect two half cells to form an electrochemical cell, electrons. The electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. Using standard electrode potentials to calculate cell emfs.
from
This kind of cell includes the galvanic, or voltaic, cell, named after. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction is called an electrochemical cell. When you connect two half cells to form an electrochemical cell, electrons. The electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. Galvanic cells and electrolytic cells. By convention, the electrode written to the left of the salt bridge in this cell notation is always taken to be the anode, and the associated half. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. Using standard electrode potentials to calculate cell emfs. There are two types of electrochemical cells:
Electrode Chemical Cell Galvanic cells and electrolytic cells. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. There are two types of electrochemical cells: By convention, the electrode written to the left of the salt bridge in this cell notation is always taken to be the anode, and the associated half. Using standard electrode potentials to calculate cell emfs. This kind of cell includes the galvanic, or voltaic, cell, named after. The electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction is called an electrochemical cell. When you connect two half cells to form an electrochemical cell, electrons. Galvanic cells and electrolytic cells.
From
Electrode Chemical Cell Using standard electrode potentials to calculate cell emfs. Galvanic cells and electrolytic cells. There are two types of electrochemical cells: An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. The electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. An electrochemical cell is a device that produces. Electrode Chemical Cell.
From
Electrode Chemical Cell There are two types of electrochemical cells: The electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction is called an electrochemical cell. By convention, the electrode written to the. Electrode Chemical Cell.
From
Electrode Chemical Cell An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. This kind of cell includes the galvanic, or voltaic, cell, named after. The electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. When you connect two half cells to form an electrochemical cell, electrons.. Electrode Chemical Cell.
From sites.google.com
Electrochemistry engineering chemistry Electrode Chemical Cell An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction is called an electrochemical cell. There are two types of electrochemical cells: When you connect two half cells to form an electrochemical cell, electrons. Galvanic cells and electrolytic cells. An electrochemical cell splits the oxidant and. Electrode Chemical Cell.
From
Electrode Chemical Cell By convention, the electrode written to the left of the salt bridge in this cell notation is always taken to be the anode, and the associated half. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. An apparatus that is used to generate electricity from a spontaneous redox reaction or,. Electrode Chemical Cell.
From
Electrode Chemical Cell The electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction is called an electrochemical cell. By convention, the electrode written to the left of the salt bridge in this. Electrode Chemical Cell.
From
Electrode Chemical Cell When you connect two half cells to form an electrochemical cell, electrons. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. The electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. There are two types of electrochemical cells: This kind of cell includes. Electrode Chemical Cell.
From
Electrode Chemical Cell Using standard electrode potentials to calculate cell emfs. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction is called an electrochemical cell. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. An electrochemical cell splits. Electrode Chemical Cell.
From www.alamy.com
Electrochemical cell or Galvanic cell, The Daniell cell with Voltmeter Electrode Chemical Cell An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. The electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. There are two types of electrochemical cells: This kind. Electrode Chemical Cell.
From www.dreamstime.com
Electrochemical Reaction Stock Illustrations 94 Electrochemical Electrode Chemical Cell An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction is called an electrochemical cell. Using standard electrode potentials to calculate cell emfs. By convention, the electrode written to the left. Electrode Chemical Cell.
From www.sigmaaldrich.com
Electrochemistry on the Bench and in the Field Electrode Chemical Cell An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction is called an electrochemical cell. An electrochemical cell is a device that produces an electric current from energy released by a. Electrode Chemical Cell.
From
Electrode Chemical Cell An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. The electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. This kind of cell includes the galvanic, or voltaic, cell, named after. There are two types of electrochemical cells: Using standard electrode potentials to calculate cell emfs. When. Electrode Chemical Cell.
From mmerevise.co.uk
Electrochemical Cells Worksheets and Revision MME Electrode Chemical Cell When you connect two half cells to form an electrochemical cell, electrons. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction is called an electrochemical cell. There are two types of electrochemical cells: An electrochemical cell splits the oxidant and reductant in a manner that. Electrode Chemical Cell.
From
Electrode Chemical Cell An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction is called an electrochemical cell. This kind of cell includes the galvanic, or voltaic, cell, named after. Using standard electrode potentials to calculate cell emfs. When you connect two half cells to form an electrochemical cell,. Electrode Chemical Cell.
From
Electrode Chemical Cell There are two types of electrochemical cells: When you connect two half cells to form an electrochemical cell, electrons. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction is called. Electrode Chemical Cell.
From
Electrode Chemical Cell The electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. There are two types of electrochemical cells: Galvanic cells and electrolytic cells. By convention, the electrode written to the left of the salt bridge in this cell notation is always taken to be the anode, and the associated half. An apparatus that is. Electrode Chemical Cell.
From ar.inspiredpencil.com
Copper Electrolytic Cell Electrode Chemical Cell An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction is called an electrochemical cell. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. There are two types of electrochemical cells: By convention, the electrode written. Electrode Chemical Cell.
From
Electrode Chemical Cell When you connect two half cells to form an electrochemical cell, electrons. There are two types of electrochemical cells: Using standard electrode potentials to calculate cell emfs. The electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. Galvanic cells and electrolytic cells. By convention, the electrode written to the left of the salt. Electrode Chemical Cell.
From classfullbooklice.z13.web.core.windows.net
Working Of Electrochemical Cell Electrode Chemical Cell An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. Galvanic cells and electrolytic cells. Using standard electrode potentials to calculate cell emfs. The electrode attached to the negative terminal of a battery is called a. Electrode Chemical Cell.
From
Electrode Chemical Cell There are two types of electrochemical cells: When you connect two half cells to form an electrochemical cell, electrons. Galvanic cells and electrolytic cells. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. An apparatus. Electrode Chemical Cell.
From
Electrode Chemical Cell An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. By convention, the electrode written to the left of the salt bridge in this cell notation is always taken to be the anode, and the associated half. The electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. There. Electrode Chemical Cell.
From saylordotorg.github.io
Electrochemistry Electrode Chemical Cell An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. The electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. This kind of cell includes the galvanic, or voltaic, cell, named after. By convention, the electrode written to the left of the salt bridge in this cell notation. Electrode Chemical Cell.
From alevelchemistry.co.uk
Electrochemical Cells Definition, Description & Types Electrode Chemical Cell By convention, the electrode written to the left of the salt bridge in this cell notation is always taken to be the anode, and the associated half. There are two types of electrochemical cells: An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. An electrochemical cell is a device that produces an electric current. Electrode Chemical Cell.
From
Electrode Chemical Cell By convention, the electrode written to the left of the salt bridge in this cell notation is always taken to be the anode, and the associated half. Using standard electrode potentials to calculate cell emfs. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. Galvanic cells and electrolytic cells. The. Electrode Chemical Cell.
From
Electrode Chemical Cell This kind of cell includes the galvanic, or voltaic, cell, named after. When you connect two half cells to form an electrochemical cell, electrons. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. Using standard electrode potentials to calculate cell emfs. By convention, the electrode written to the left of the salt bridge in. Electrode Chemical Cell.
From www.thoughtco.com
Electrochemical Cell Definition Electrode Chemical Cell An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. When you connect two half cells to form an electrochemical cell, electrons. This kind of cell includes the galvanic, or voltaic, cell, named after. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses. Electrode Chemical Cell.
From
Electrode Chemical Cell An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction is called an electrochemical cell. Galvanic cells and electrolytic cells. Using standard electrode potentials to calculate cell emfs. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox. Electrode Chemical Cell.
From
Electrode Chemical Cell Galvanic cells and electrolytic cells. There are two types of electrochemical cells: An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction is called an electrochemical cell. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. By convention, the electrode. Electrode Chemical Cell.
From
Electrode Chemical Cell The electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. There are two types of electrochemical cells: An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction is called an electrochemical cell. An electrochemical cell splits the oxidant and. Electrode Chemical Cell.
From
Electrode Chemical Cell Galvanic cells and electrolytic cells. Using standard electrode potentials to calculate cell emfs. There are two types of electrochemical cells: This kind of cell includes the galvanic, or voltaic, cell, named after. By convention, the electrode written to the left of the salt bridge in this cell notation is always taken to be the anode, and the associated half. An. Electrode Chemical Cell.
From
Electrode Chemical Cell An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction is called an electrochemical cell. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. This kind of cell includes the galvanic, or voltaic, cell, named after. When you connect two. Electrode Chemical Cell.
From
Electrode Chemical Cell Using standard electrode potentials to calculate cell emfs. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. The electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. When you connect two half cells to form an electrochemical cell, electrons. By convention, the electrode written to the left. Electrode Chemical Cell.
From
Electrode Chemical Cell An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. This kind of cell includes the galvanic, or voltaic, cell, named after. When you connect two half cells to form an electrochemical cell, electrons. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a. Electrode Chemical Cell.
From
Electrode Chemical Cell An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction is called an electrochemical cell. The electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. An electrochemical cell is a device that produces an electric current from energy released. Electrode Chemical Cell.
From chem.libretexts.org
11.1 Galvanic Cells Chemistry LibreTexts Electrode Chemical Cell Using standard electrode potentials to calculate cell emfs. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction is called an electrochemical cell. Galvanic cells and electrolytic cells. When you connect two half cells to form an electrochemical cell, electrons. An electrochemical cell splits the oxidant. Electrode Chemical Cell.