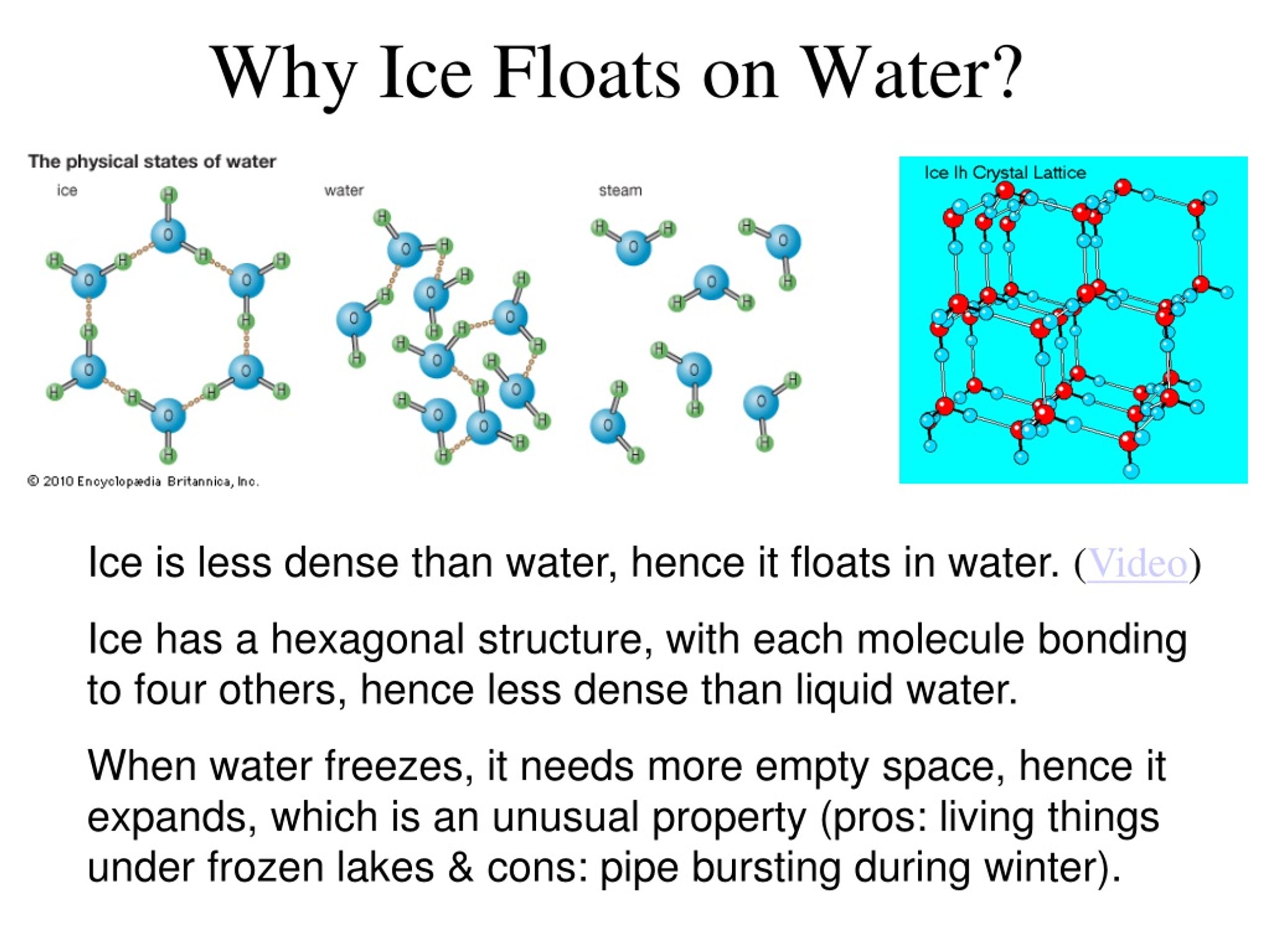
Why Does Ice Float Gcse . Therefore, ice floats on water simply because it is less dense than water. The heavier water displaces the lighter ice, so ice floats to the top. Ice floats because it is about 9% less dense than liquid water. Water is unlike other substances in terms of changes in density. Discover the fascinating science behind why ice floats! The structure of ice contributes to its floating because of its unique molecular arrangement. 🌬️ ️ dive into the crystalline structure of. A water molecule (h2o) is made of two hydrogen atoms and one oxygen atom. In ice, water molecules form a hexagonal lattice that creates more space between molecules compared to the more compact arrangement in liquid water. Why does ice float on water. This expanded structure decreases the density of ice, allowing it to float on the denser. But why does ice float on water? It is common for us to observe ice cubes floating when placed in a glass of water, and icebergs floating on the surface seas and oceans. Ice is the only common solid that floats on its liquid form. An object less dense than water will float.
from www.slideserve.com
The hydrogen and oxygen atoms share electron pairs, forming. Ice is the only common solid that floats on its liquid form. The structure of ice contributes to its floating because of its unique molecular arrangement. In fact, why does anything float at all? It is common for us to observe ice cubes floating when placed in a glass of water, and icebergs floating on the surface seas and oceans. Professor brian cox explains it's all down to a hidden force of nature. Ice cubes float because of their molecular structure. In other words, ice takes up about 9% more space than water, so a liter of ice weighs less than liter water. Therefore, ice floats on water simply because it is less dense than water. Discover the fascinating science behind why ice floats!
PPT Chapter 11 Fluid Statics PowerPoint Presentation, free download
Why Does Ice Float Gcse In ice, water molecules form a hexagonal lattice that creates more space between molecules compared to the more compact arrangement in liquid water. But why does ice float on water? The heavier water displaces the lighter ice, so ice floats to the top. Professor brian cox explains it's all down to a hidden force of nature. In ice, water molecules form a hexagonal lattice that creates more space between molecules compared to the more compact arrangement in liquid water. A water molecule (h2o) is made of two hydrogen atoms and one oxygen atom. In fact, why does anything float at all? It is common for us to observe ice cubes floating when placed in a glass of water, and icebergs floating on the surface seas and oceans. Why does ice float on water. Discover the fascinating science behind why ice floats! An object less dense than water will float. The hydrogen and oxygen atoms share electron pairs, forming. Water is unlike other substances in terms of changes in density. 🌬️ ️ dive into the crystalline structure of. Therefore, ice floats on water simply because it is less dense than water. Ice cubes float because of their molecular structure.
From www.slideserve.com
PPT Ch 1 The Chemistry of Life PowerPoint Presentation, free Why Does Ice Float Gcse Ice is the only common solid that floats on its liquid form. In ice, water molecules form a hexagonal lattice that creates more space between molecules compared to the more compact arrangement in liquid water. Water is unlike other substances in terms of changes in density. Professor brian cox explains it's all down to a hidden force of nature. Discover. Why Does Ice Float Gcse.
From www.teachoo.com
Liquids generally have lower density as compared to solids. But you Why Does Ice Float Gcse Why does ice float on water. The structure of ice contributes to its floating because of its unique molecular arrangement. A water molecule (h2o) is made of two hydrogen atoms and one oxygen atom. Ice is the only common solid that floats on its liquid form. Discover the fascinating science behind why ice floats! Water is unlike other substances in. Why Does Ice Float Gcse.
From learnglassblowing.com
Why Ice Cubes Float In Water Learn Glass Blowing Why Does Ice Float Gcse A water molecule (h2o) is made of two hydrogen atoms and one oxygen atom. The structure of ice contributes to its floating because of its unique molecular arrangement. Why does ice float on water. Ice is the only common solid that floats on its liquid form. An object less dense than water will float. The hydrogen and oxygen atoms share. Why Does Ice Float Gcse.
From www.youtube.com
Why Ice Floats on Water YouTube Why Does Ice Float Gcse Ice cubes float because of their molecular structure. It is common for us to observe ice cubes floating when placed in a glass of water, and icebergs floating on the surface seas and oceans. In fact, why does anything float at all? Why does ice float on water. But why does ice float on water? Therefore, ice floats on water. Why Does Ice Float Gcse.
From www.youtube.com
Why does ice float above water? / Optical Knowledge/ Science in every Why Does Ice Float Gcse The heavier water displaces the lighter ice, so ice floats to the top. Ice is the only common solid that floats on its liquid form. An object less dense than water will float. Ice cubes float because of their molecular structure. Discover the fascinating science behind why ice floats! The hydrogen and oxygen atoms share electron pairs, forming. The structure. Why Does Ice Float Gcse.
From slidesharenow.blogspot.com
Why Does Ice Float On Water slideshare Why Does Ice Float Gcse A water molecule (h2o) is made of two hydrogen atoms and one oxygen atom. The hydrogen and oxygen atoms share electron pairs, forming. Therefore, ice floats on water simply because it is less dense than water. This expanded structure decreases the density of ice, allowing it to float on the denser. 🌬️ ️ dive into the crystalline structure of. In. Why Does Ice Float Gcse.
From dokumen.tips
(PPT) BW Why does ice float in water? BW Why does ice float in water Why Does Ice Float Gcse An object less dense than water will float. It is common for us to observe ice cubes floating when placed in a glass of water, and icebergs floating on the surface seas and oceans. Professor brian cox explains it's all down to a hidden force of nature. Ice is the only common solid that floats on its liquid form. The. Why Does Ice Float Gcse.
From academichelp.net
Why Does Ice Float on Water? The Science Behind It Explained Why Does Ice Float Gcse The heavier water displaces the lighter ice, so ice floats to the top. A water molecule (h2o) is made of two hydrogen atoms and one oxygen atom. Why does ice float on water. Ice cubes float because of their molecular structure. In fact, why does anything float at all? This expanded structure decreases the density of ice, allowing it to. Why Does Ice Float Gcse.
From www.slideserve.com
PPT Chapter 11 Fluid Statics PowerPoint Presentation, free download Why Does Ice Float Gcse Professor brian cox explains it's all down to a hidden force of nature. In ice, water molecules form a hexagonal lattice that creates more space between molecules compared to the more compact arrangement in liquid water. Discover the fascinating science behind why ice floats! The hydrogen and oxygen atoms share electron pairs, forming. 🌬️ ️ dive into the crystalline structure. Why Does Ice Float Gcse.
From www.slideserve.com
PPT Water PowerPoint Presentation, free download ID1344926 Why Does Ice Float Gcse This expanded structure decreases the density of ice, allowing it to float on the denser. In ice, water molecules form a hexagonal lattice that creates more space between molecules compared to the more compact arrangement in liquid water. In other words, ice takes up about 9% more space than water, so a liter of ice weighs less than liter water.. Why Does Ice Float Gcse.
From www.slideserve.com
PPT Chapter 17 Water and Aqueous Systems PowerPoint Presentation Why Does Ice Float Gcse In other words, ice takes up about 9% more space than water, so a liter of ice weighs less than liter water. But why does ice float on water? In ice, water molecules form a hexagonal lattice that creates more space between molecules compared to the more compact arrangement in liquid water. Ice cubes float because of their molecular structure.. Why Does Ice Float Gcse.
From www.youtube.com
Why ice floats on liquid water? Why volume of water increases on Why Does Ice Float Gcse In ice, water molecules form a hexagonal lattice that creates more space between molecules compared to the more compact arrangement in liquid water. Professor brian cox explains it's all down to a hidden force of nature. The structure of ice contributes to its floating because of its unique molecular arrangement. In fact, why does anything float at all? Therefore, ice. Why Does Ice Float Gcse.
From www.thoughtco.com
Why Does Ice Float? Ice and the Density of Water Why Does Ice Float Gcse Why does ice float on water. In ice, water molecules form a hexagonal lattice that creates more space between molecules compared to the more compact arrangement in liquid water. An object less dense than water will float. Discover the fascinating science behind why ice floats! It is common for us to observe ice cubes floating when placed in a glass. Why Does Ice Float Gcse.
From www.slideserve.com
PPT L14 Fluids [3] PowerPoint Presentation, free download ID6186946 Why Does Ice Float Gcse The heavier water displaces the lighter ice, so ice floats to the top. Professor brian cox explains it's all down to a hidden force of nature. Ice cubes float because of their molecular structure. Therefore, ice floats on water simply because it is less dense than water. The structure of ice contributes to its floating because of its unique molecular. Why Does Ice Float Gcse.
From www.slideserve.com
PPT Why Ice Floats PowerPoint Presentation, free download ID9719781 Why Does Ice Float Gcse The hydrogen and oxygen atoms share electron pairs, forming. In fact, why does anything float at all? Ice floats because it is about 9% less dense than liquid water. Why does ice float on water. Discover the fascinating science behind why ice floats! 🌬️ ️ dive into the crystalline structure of. A water molecule (h2o) is made of two hydrogen. Why Does Ice Float Gcse.
From www.slideserve.com
PPT Appetizer 1/14/13 PowerPoint Presentation, free download ID Why Does Ice Float Gcse In ice, water molecules form a hexagonal lattice that creates more space between molecules compared to the more compact arrangement in liquid water. The heavier water displaces the lighter ice, so ice floats to the top. In other words, ice takes up about 9% more space than water, so a liter of ice weighs less than liter water. But why. Why Does Ice Float Gcse.
From zakruti.com
Why does ice float in water? Zaidan and Charles Morton Why Does Ice Float Gcse In other words, ice takes up about 9% more space than water, so a liter of ice weighs less than liter water. In ice, water molecules form a hexagonal lattice that creates more space between molecules compared to the more compact arrangement in liquid water. Therefore, ice floats on water simply because it is less dense than water. Discover the. Why Does Ice Float Gcse.
From www.dreamstime.com
Why Does Ice Float on Water Infographic Diagram Stock Vector Why Does Ice Float Gcse An object less dense than water will float. It is common for us to observe ice cubes floating when placed in a glass of water, and icebergs floating on the surface seas and oceans. This expanded structure decreases the density of ice, allowing it to float on the denser. In other words, ice takes up about 9% more space than. Why Does Ice Float Gcse.
From www.slideserve.com
PPT The Chemistry of Life PowerPoint Presentation, free download ID Why Does Ice Float Gcse Ice floats because it is about 9% less dense than liquid water. But why does ice float on water? Therefore, ice floats on water simply because it is less dense than water. 🌬️ ️ dive into the crystalline structure of. Ice cubes float because of their molecular structure. A water molecule (h2o) is made of two hydrogen atoms and one. Why Does Ice Float Gcse.
From techiescientist.com
Why Does Ice Float on Water? Techiescientist Why Does Ice Float Gcse Discover the fascinating science behind why ice floats! In fact, why does anything float at all? It is common for us to observe ice cubes floating when placed in a glass of water, and icebergs floating on the surface seas and oceans. This expanded structure decreases the density of ice, allowing it to float on the denser. 🌬️ ️ dive. Why Does Ice Float Gcse.
From www.youtube.com
Why ICE FLOATS on WATER YouTube Why Does Ice Float Gcse 🌬️ ️ dive into the crystalline structure of. In other words, ice takes up about 9% more space than water, so a liter of ice weighs less than liter water. A water molecule (h2o) is made of two hydrogen atoms and one oxygen atom. Therefore, ice floats on water simply because it is less dense than water. Water is unlike. Why Does Ice Float Gcse.
From www.slideshare.net
Why does ice float on water? Why Does Ice Float Gcse In other words, ice takes up about 9% more space than water, so a liter of ice weighs less than liter water. Ice cubes float because of their molecular structure. Therefore, ice floats on water simply because it is less dense than water. A water molecule (h2o) is made of two hydrogen atoms and one oxygen atom. Why does ice. Why Does Ice Float Gcse.
From www.youtube.com
Why does ice(/ice cubes) float on water? தமிழ் Samy22 YouTube Why Does Ice Float Gcse The heavier water displaces the lighter ice, so ice floats to the top. Discover the fascinating science behind why ice floats! 🌬️ ️ dive into the crystalline structure of. This expanded structure decreases the density of ice, allowing it to float on the denser. Professor brian cox explains it's all down to a hidden force of nature. In fact, why. Why Does Ice Float Gcse.
From www.slideshare.net
Why does ice float Why Does Ice Float Gcse In other words, ice takes up about 9% more space than water, so a liter of ice weighs less than liter water. The hydrogen and oxygen atoms share electron pairs, forming. 🌬️ ️ dive into the crystalline structure of. A water molecule (h2o) is made of two hydrogen atoms and one oxygen atom. This expanded structure decreases the density of. Why Does Ice Float Gcse.
From www.slideserve.com
PPT L14 Fluids [3] PowerPoint Presentation, free download ID5631091 Why Does Ice Float Gcse Why does ice float on water. This expanded structure decreases the density of ice, allowing it to float on the denser. In ice, water molecules form a hexagonal lattice that creates more space between molecules compared to the more compact arrangement in liquid water. The structure of ice contributes to its floating because of its unique molecular arrangement. But why. Why Does Ice Float Gcse.
From answerdbmaligner.z21.web.core.windows.net
How Does Ice Float Why Does Ice Float Gcse Water is unlike other substances in terms of changes in density. Ice is the only common solid that floats on its liquid form. Why does ice float on water. But why does ice float on water? The hydrogen and oxygen atoms share electron pairs, forming. Therefore, ice floats on water simply because it is less dense than water. In other. Why Does Ice Float Gcse.
From higheducations.com
Why Does Ice Float in Liquid Water? Why Does Ice Float Gcse It is common for us to observe ice cubes floating when placed in a glass of water, and icebergs floating on the surface seas and oceans. Therefore, ice floats on water simply because it is less dense than water. The hydrogen and oxygen atoms share electron pairs, forming. Why does ice float on water. Professor brian cox explains it's all. Why Does Ice Float Gcse.
From www.slideserve.com
PPT Aim How do we calculate density? PowerPoint Presentation, free Why Does Ice Float Gcse Ice is the only common solid that floats on its liquid form. Why does ice float on water. 🌬️ ️ dive into the crystalline structure of. This expanded structure decreases the density of ice, allowing it to float on the denser. An object less dense than water will float. Professor brian cox explains it's all down to a hidden force. Why Does Ice Float Gcse.
From slideplayer.com
Water Unit. ppt download Why Does Ice Float Gcse Discover the fascinating science behind why ice floats! Ice is the only common solid that floats on its liquid form. In other words, ice takes up about 9% more space than water, so a liter of ice weighs less than liter water. Water is unlike other substances in terms of changes in density. In fact, why does anything float at. Why Does Ice Float Gcse.
From issuu.com
Why Does Ice Float On Water Chemistry? by AmericanLifeguardManual Issuu Why Does Ice Float Gcse In ice, water molecules form a hexagonal lattice that creates more space between molecules compared to the more compact arrangement in liquid water. A water molecule (h2o) is made of two hydrogen atoms and one oxygen atom. In fact, why does anything float at all? Discover the fascinating science behind why ice floats! Professor brian cox explains it's all down. Why Does Ice Float Gcse.
From www.worldatlas.com
Why Does Ice Float? WorldAtlas Why Does Ice Float Gcse But why does ice float on water? The hydrogen and oxygen atoms share electron pairs, forming. Ice floats because it is about 9% less dense than liquid water. The heavier water displaces the lighter ice, so ice floats to the top. Professor brian cox explains it's all down to a hidden force of nature. The structure of ice contributes to. Why Does Ice Float Gcse.
From www.youtube.com
why does ice float on the water?? easylearning4all YouTube Why Does Ice Float Gcse The heavier water displaces the lighter ice, so ice floats to the top. In ice, water molecules form a hexagonal lattice that creates more space between molecules compared to the more compact arrangement in liquid water. Why does ice float on water. Discover the fascinating science behind why ice floats! Ice floats because it is about 9% less dense than. Why Does Ice Float Gcse.
From www.livescience.com
Why does ice float? Live Science Why Does Ice Float Gcse It is common for us to observe ice cubes floating when placed in a glass of water, and icebergs floating on the surface seas and oceans. Ice floats because it is about 9% less dense than liquid water. Ice cubes float because of their molecular structure. Discover the fascinating science behind why ice floats! The structure of ice contributes to. Why Does Ice Float Gcse.
From thevigyan.com
Why Does Ice Float in Water? The Vigyan Why Does Ice Float Gcse 🌬️ ️ dive into the crystalline structure of. It is common for us to observe ice cubes floating when placed in a glass of water, and icebergs floating on the surface seas and oceans. This expanded structure decreases the density of ice, allowing it to float on the denser. But why does ice float on water? Professor brian cox explains. Why Does Ice Float Gcse.
From www.youtube.com
Why does ice float on water? Detailed Explanation YouTube Why Does Ice Float Gcse The structure of ice contributes to its floating because of its unique molecular arrangement. Discover the fascinating science behind why ice floats! An object less dense than water will float. In fact, why does anything float at all? Water is unlike other substances in terms of changes in density. The hydrogen and oxygen atoms share electron pairs, forming. 🌬️ ️. Why Does Ice Float Gcse.