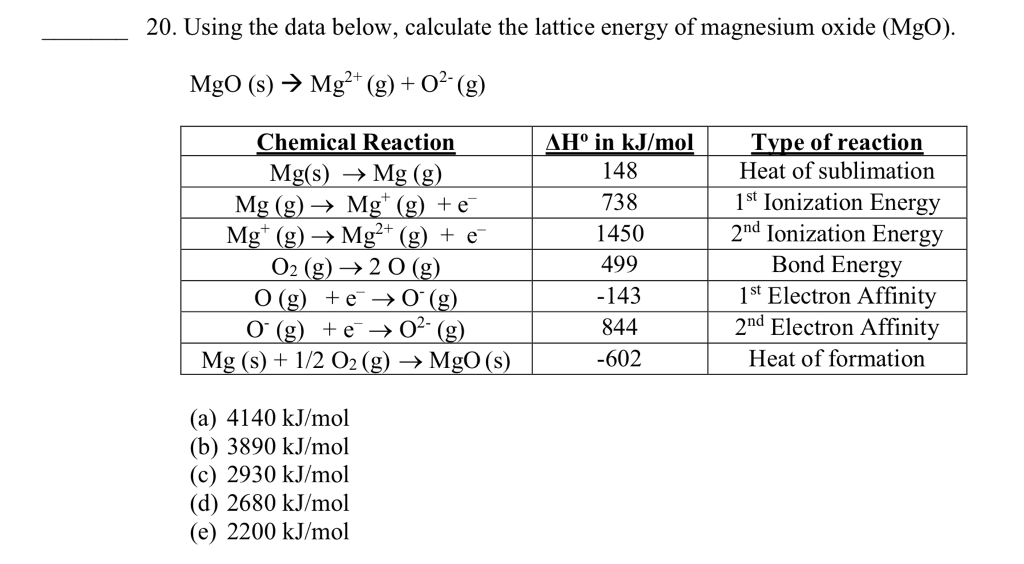
Magnesium Oxide Lattice Energy . The lattice enthalpy of magnesium oxide is also increased relative to sodium chloride because magnesium ions are smaller than sodium ions,. Mg 2+ (g) + o 2. \(u\) can be calculated from the charges on. The lattice energy (\(u\)) of an ionic substance is defined as the energy required to dissociate the solid into gaseous ions; That is because there are stronger ionic. You need to put in more energy to ionize the magnesium to give a 2+ ion, but a lot more energy is released as lattice enthalpy. You need to put in more energy to ionise the magnesium to give a 2+ ion, but a lot more energy is released as lattice enthalpy.
from www.chegg.com
\(u\) can be calculated from the charges on. The lattice energy (\(u\)) of an ionic substance is defined as the energy required to dissociate the solid into gaseous ions; You need to put in more energy to ionize the magnesium to give a 2+ ion, but a lot more energy is released as lattice enthalpy. That is because there are stronger ionic. You need to put in more energy to ionise the magnesium to give a 2+ ion, but a lot more energy is released as lattice enthalpy. Mg 2+ (g) + o 2. The lattice enthalpy of magnesium oxide is also increased relative to sodium chloride because magnesium ions are smaller than sodium ions,.
Solved 20. Using the data below, calculate the lattice
Magnesium Oxide Lattice Energy That is because there are stronger ionic. You need to put in more energy to ionize the magnesium to give a 2+ ion, but a lot more energy is released as lattice enthalpy. The lattice energy (\(u\)) of an ionic substance is defined as the energy required to dissociate the solid into gaseous ions; That is because there are stronger ionic. The lattice enthalpy of magnesium oxide is also increased relative to sodium chloride because magnesium ions are smaller than sodium ions,. Mg 2+ (g) + o 2. You need to put in more energy to ionise the magnesium to give a 2+ ion, but a lot more energy is released as lattice enthalpy. \(u\) can be calculated from the charges on.
From www.chegg.com
Solved 20. Using the data below, calculate the lattice Magnesium Oxide Lattice Energy Mg 2+ (g) + o 2. You need to put in more energy to ionise the magnesium to give a 2+ ion, but a lot more energy is released as lattice enthalpy. You need to put in more energy to ionize the magnesium to give a 2+ ion, but a lot more energy is released as lattice enthalpy. The lattice. Magnesium Oxide Lattice Energy.
From www.slideserve.com
PPT Lattice enthalpy PowerPoint Presentation, free download ID6559902 Magnesium Oxide Lattice Energy That is because there are stronger ionic. \(u\) can be calculated from the charges on. The lattice enthalpy of magnesium oxide is also increased relative to sodium chloride because magnesium ions are smaller than sodium ions,. You need to put in more energy to ionise the magnesium to give a 2+ ion, but a lot more energy is released as. Magnesium Oxide Lattice Energy.
From www.youtube.com
Ch11 Lec3 Determination of Lattice energy of Magnesium Oxide (MgO Magnesium Oxide Lattice Energy You need to put in more energy to ionize the magnesium to give a 2+ ion, but a lot more energy is released as lattice enthalpy. You need to put in more energy to ionise the magnesium to give a 2+ ion, but a lot more energy is released as lattice enthalpy. Mg 2+ (g) + o 2. The lattice. Magnesium Oxide Lattice Energy.
From www.pathwaystochemistry.com
Formation of Ionic Compounds The Born Haber Cycle Pathways to Chemistry Magnesium Oxide Lattice Energy \(u\) can be calculated from the charges on. That is because there are stronger ionic. The lattice enthalpy of magnesium oxide is also increased relative to sodium chloride because magnesium ions are smaller than sodium ions,. You need to put in more energy to ionize the magnesium to give a 2+ ion, but a lot more energy is released as. Magnesium Oxide Lattice Energy.
From ar.inspiredpencil.com
Magnesium Oxide Lattice Structure Magnesium Oxide Lattice Energy You need to put in more energy to ionise the magnesium to give a 2+ ion, but a lot more energy is released as lattice enthalpy. That is because there are stronger ionic. \(u\) can be calculated from the charges on. The lattice enthalpy of magnesium oxide is also increased relative to sodium chloride because magnesium ions are smaller than. Magnesium Oxide Lattice Energy.
From www.youtube.com
Lattice Enthalpy and Born Haber Cycle of Magnesium Oxide YouTube Magnesium Oxide Lattice Energy The lattice energy (\(u\)) of an ionic substance is defined as the energy required to dissociate the solid into gaseous ions; \(u\) can be calculated from the charges on. You need to put in more energy to ionise the magnesium to give a 2+ ion, but a lot more energy is released as lattice enthalpy. Mg 2+ (g) + o. Magnesium Oxide Lattice Energy.
From www.researchgate.net
(a) Unit cell of the magnesium structure, (b) the reciprocal lattice Magnesium Oxide Lattice Energy \(u\) can be calculated from the charges on. Mg 2+ (g) + o 2. You need to put in more energy to ionise the magnesium to give a 2+ ion, but a lot more energy is released as lattice enthalpy. That is because there are stronger ionic. You need to put in more energy to ionize the magnesium to give. Magnesium Oxide Lattice Energy.
From ar.inspiredpencil.com
Magnesium Oxide Lattice Structure Magnesium Oxide Lattice Energy \(u\) can be calculated from the charges on. You need to put in more energy to ionise the magnesium to give a 2+ ion, but a lot more energy is released as lattice enthalpy. The lattice energy (\(u\)) of an ionic substance is defined as the energy required to dissociate the solid into gaseous ions; That is because there are. Magnesium Oxide Lattice Energy.
From ar.inspiredpencil.com
Magnesium Oxide Lattice Structure Magnesium Oxide Lattice Energy Mg 2+ (g) + o 2. You need to put in more energy to ionise the magnesium to give a 2+ ion, but a lot more energy is released as lattice enthalpy. That is because there are stronger ionic. You need to put in more energy to ionize the magnesium to give a 2+ ion, but a lot more energy. Magnesium Oxide Lattice Energy.
From www.thesciencehive.co.uk
Lattice Enthalpy* — the science sauce Magnesium Oxide Lattice Energy The lattice energy (\(u\)) of an ionic substance is defined as the energy required to dissociate the solid into gaseous ions; That is because there are stronger ionic. The lattice enthalpy of magnesium oxide is also increased relative to sodium chloride because magnesium ions are smaller than sodium ions,. You need to put in more energy to ionize the magnesium. Magnesium Oxide Lattice Energy.
From ar.inspiredpencil.com
Magnesium Oxide Lattice Structure Magnesium Oxide Lattice Energy Mg 2+ (g) + o 2. That is because there are stronger ionic. The lattice enthalpy of magnesium oxide is also increased relative to sodium chloride because magnesium ions are smaller than sodium ions,. You need to put in more energy to ionize the magnesium to give a 2+ ion, but a lot more energy is released as lattice enthalpy.. Magnesium Oxide Lattice Energy.
From goodttorials.blogspot.com
How To Find Lattice Energy Born Haber Cycle Magnesium Oxide Lattice Energy You need to put in more energy to ionize the magnesium to give a 2+ ion, but a lot more energy is released as lattice enthalpy. That is because there are stronger ionic. You need to put in more energy to ionise the magnesium to give a 2+ ion, but a lot more energy is released as lattice enthalpy. The. Magnesium Oxide Lattice Energy.
From www.alamy.com
BornHaber cycle calculation of ionic crystal lattice energy Stock Magnesium Oxide Lattice Energy \(u\) can be calculated from the charges on. The lattice enthalpy of magnesium oxide is also increased relative to sodium chloride because magnesium ions are smaller than sodium ions,. That is because there are stronger ionic. The lattice energy (\(u\)) of an ionic substance is defined as the energy required to dissociate the solid into gaseous ions; Mg 2+ (g). Magnesium Oxide Lattice Energy.
From ar.inspiredpencil.com
Magnesium Oxide Lattice Structure Magnesium Oxide Lattice Energy Mg 2+ (g) + o 2. The lattice enthalpy of magnesium oxide is also increased relative to sodium chloride because magnesium ions are smaller than sodium ions,. You need to put in more energy to ionize the magnesium to give a 2+ ion, but a lot more energy is released as lattice enthalpy. You need to put in more energy. Magnesium Oxide Lattice Energy.
From www.youtube.com
Lattice Energies Chemistry Tutorial YouTube Magnesium Oxide Lattice Energy That is because there are stronger ionic. The lattice enthalpy of magnesium oxide is also increased relative to sodium chloride because magnesium ions are smaller than sodium ions,. You need to put in more energy to ionize the magnesium to give a 2+ ion, but a lot more energy is released as lattice enthalpy. The lattice energy (\(u\)) of an. Magnesium Oxide Lattice Energy.
From ar.inspiredpencil.com
Magnesium Oxide Lattice Structure Magnesium Oxide Lattice Energy The lattice enthalpy of magnesium oxide is also increased relative to sodium chloride because magnesium ions are smaller than sodium ions,. The lattice energy (\(u\)) of an ionic substance is defined as the energy required to dissociate the solid into gaseous ions; You need to put in more energy to ionise the magnesium to give a 2+ ion, but a. Magnesium Oxide Lattice Energy.
From www.animalia-life.club
Lattice Energy Equation Magnesium Oxide Lattice Energy You need to put in more energy to ionise the magnesium to give a 2+ ion, but a lot more energy is released as lattice enthalpy. You need to put in more energy to ionize the magnesium to give a 2+ ion, but a lot more energy is released as lattice enthalpy. The lattice energy (\(u\)) of an ionic substance. Magnesium Oxide Lattice Energy.
From saylordotorg.github.io
Lattice Energies in Ionic Solids Magnesium Oxide Lattice Energy Mg 2+ (g) + o 2. \(u\) can be calculated from the charges on. The lattice enthalpy of magnesium oxide is also increased relative to sodium chloride because magnesium ions are smaller than sodium ions,. You need to put in more energy to ionize the magnesium to give a 2+ ion, but a lot more energy is released as lattice. Magnesium Oxide Lattice Energy.
From www.slideserve.com
PPT Lattice enthalpy PowerPoint Presentation, free download ID6559902 Magnesium Oxide Lattice Energy \(u\) can be calculated from the charges on. That is because there are stronger ionic. The lattice energy (\(u\)) of an ionic substance is defined as the energy required to dissociate the solid into gaseous ions; You need to put in more energy to ionise the magnesium to give a 2+ ion, but a lot more energy is released as. Magnesium Oxide Lattice Energy.
From www.youtube.com
Formation of Magnesium Oxide (MgO) Chemical Bonding Atomic Magnesium Oxide Lattice Energy \(u\) can be calculated from the charges on. The lattice energy (\(u\)) of an ionic substance is defined as the energy required to dissociate the solid into gaseous ions; That is because there are stronger ionic. You need to put in more energy to ionize the magnesium to give a 2+ ion, but a lot more energy is released as. Magnesium Oxide Lattice Energy.
From ar.inspiredpencil.com
Magnesium Oxide Lattice Structure Magnesium Oxide Lattice Energy \(u\) can be calculated from the charges on. Mg 2+ (g) + o 2. The lattice energy (\(u\)) of an ionic substance is defined as the energy required to dissociate the solid into gaseous ions; That is because there are stronger ionic. You need to put in more energy to ionize the magnesium to give a 2+ ion, but a. Magnesium Oxide Lattice Energy.
From goodttorials.blogspot.com
How To Find Lattice Energy Born Haber Cycle Magnesium Oxide Lattice Energy You need to put in more energy to ionise the magnesium to give a 2+ ion, but a lot more energy is released as lattice enthalpy. The lattice enthalpy of magnesium oxide is also increased relative to sodium chloride because magnesium ions are smaller than sodium ions,. You need to put in more energy to ionize the magnesium to give. Magnesium Oxide Lattice Energy.
From chemizi.blogspot.com
Lattice energy, BornLande equation and BornHaber cycle . Magnesium Oxide Lattice Energy That is because there are stronger ionic. The lattice energy (\(u\)) of an ionic substance is defined as the energy required to dissociate the solid into gaseous ions; You need to put in more energy to ionize the magnesium to give a 2+ ion, but a lot more energy is released as lattice enthalpy. You need to put in more. Magnesium Oxide Lattice Energy.
From chem-trip-web.blogspot.com
Understanding Electrovalent Bonds Formation, Types, Properties, and Magnesium Oxide Lattice Energy That is because there are stronger ionic. \(u\) can be calculated from the charges on. You need to put in more energy to ionize the magnesium to give a 2+ ion, but a lot more energy is released as lattice enthalpy. Mg 2+ (g) + o 2. You need to put in more energy to ionise the magnesium to give. Magnesium Oxide Lattice Energy.
From ar.inspiredpencil.com
Magnesium Oxide Lattice Structure Magnesium Oxide Lattice Energy You need to put in more energy to ionise the magnesium to give a 2+ ion, but a lot more energy is released as lattice enthalpy. \(u\) can be calculated from the charges on. That is because there are stronger ionic. The lattice energy (\(u\)) of an ionic substance is defined as the energy required to dissociate the solid into. Magnesium Oxide Lattice Energy.
From saylordotorg.github.io
Lattice Energies in Ionic Solids Magnesium Oxide Lattice Energy You need to put in more energy to ionize the magnesium to give a 2+ ion, but a lot more energy is released as lattice enthalpy. The lattice enthalpy of magnesium oxide is also increased relative to sodium chloride because magnesium ions are smaller than sodium ions,. Mg 2+ (g) + o 2. The lattice energy (\(u\)) of an ionic. Magnesium Oxide Lattice Energy.
From www.numerade.com
SOLVEDCalculate the lattice energy for magnesium oxide using both the Magnesium Oxide Lattice Energy The lattice enthalpy of magnesium oxide is also increased relative to sodium chloride because magnesium ions are smaller than sodium ions,. You need to put in more energy to ionize the magnesium to give a 2+ ion, but a lot more energy is released as lattice enthalpy. That is because there are stronger ionic. You need to put in more. Magnesium Oxide Lattice Energy.
From solidstateelectrochemistry.blogspot.com
SOLID STATE ELECTROCHEMISTRY LATTICE ENERGY_1 Magnesium Oxide Lattice Energy You need to put in more energy to ionise the magnesium to give a 2+ ion, but a lot more energy is released as lattice enthalpy. Mg 2+ (g) + o 2. That is because there are stronger ionic. \(u\) can be calculated from the charges on. The lattice energy (\(u\)) of an ionic substance is defined as the energy. Magnesium Oxide Lattice Energy.
From www.researchgate.net
(a) Perspective view of the MgO crystalline structure. The unit cell is Magnesium Oxide Lattice Energy The lattice energy (\(u\)) of an ionic substance is defined as the energy required to dissociate the solid into gaseous ions; \(u\) can be calculated from the charges on. Mg 2+ (g) + o 2. You need to put in more energy to ionise the magnesium to give a 2+ ion, but a lot more energy is released as lattice. Magnesium Oxide Lattice Energy.
From ar.inspiredpencil.com
Magnesium Oxide Lattice Structure Magnesium Oxide Lattice Energy The lattice energy (\(u\)) of an ionic substance is defined as the energy required to dissociate the solid into gaseous ions; \(u\) can be calculated from the charges on. You need to put in more energy to ionise the magnesium to give a 2+ ion, but a lot more energy is released as lattice enthalpy. You need to put in. Magnesium Oxide Lattice Energy.
From www.youtube.com
Lattice Enthalpy and Born Haber Cycle for Magnesium Chloride YouTube Magnesium Oxide Lattice Energy You need to put in more energy to ionize the magnesium to give a 2+ ion, but a lot more energy is released as lattice enthalpy. You need to put in more energy to ionise the magnesium to give a 2+ ion, but a lot more energy is released as lattice enthalpy. The lattice energy (\(u\)) of an ionic substance. Magnesium Oxide Lattice Energy.
From isaacphysics.org
Isaac Physics Magnesium Oxide Lattice Energy You need to put in more energy to ionize the magnesium to give a 2+ ion, but a lot more energy is released as lattice enthalpy. That is because there are stronger ionic. You need to put in more energy to ionise the magnesium to give a 2+ ion, but a lot more energy is released as lattice enthalpy. Mg. Magnesium Oxide Lattice Energy.
From www.thesciencehive.co.uk
Lattice Enthalpy* — the science sauce Magnesium Oxide Lattice Energy The lattice enthalpy of magnesium oxide is also increased relative to sodium chloride because magnesium ions are smaller than sodium ions,. The lattice energy (\(u\)) of an ionic substance is defined as the energy required to dissociate the solid into gaseous ions; You need to put in more energy to ionise the magnesium to give a 2+ ion, but a. Magnesium Oxide Lattice Energy.
From ar.inspiredpencil.com
Magnesium Oxide Lattice Structure Magnesium Oxide Lattice Energy You need to put in more energy to ionize the magnesium to give a 2+ ion, but a lot more energy is released as lattice enthalpy. The lattice energy (\(u\)) of an ionic substance is defined as the energy required to dissociate the solid into gaseous ions; That is because there are stronger ionic. Mg 2+ (g) + o 2.. Magnesium Oxide Lattice Energy.
From www.animalia-life.club
Lattice Energy Equation Magnesium Oxide Lattice Energy \(u\) can be calculated from the charges on. The lattice enthalpy of magnesium oxide is also increased relative to sodium chloride because magnesium ions are smaller than sodium ions,. The lattice energy (\(u\)) of an ionic substance is defined as the energy required to dissociate the solid into gaseous ions; You need to put in more energy to ionize the. Magnesium Oxide Lattice Energy.