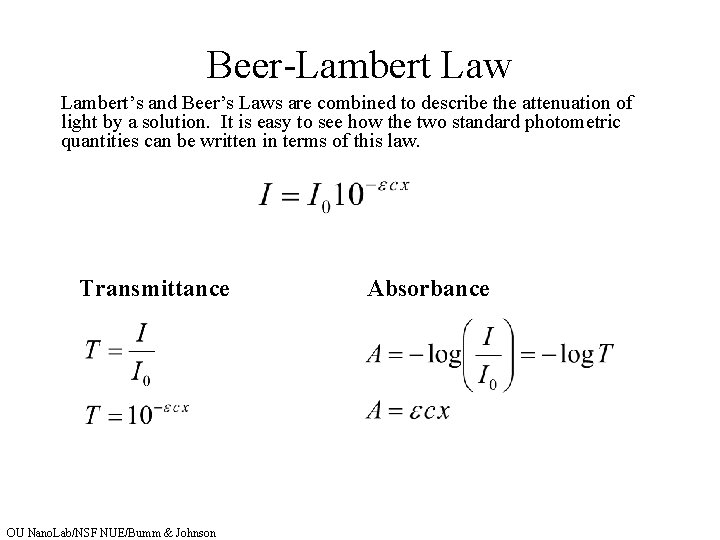
Beer Lambert Law Explained . It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. Beer's law states that the absorbance is proportional to the concentration of the sample. The law is simply an application of the observation that, within certain ranges, the absorbance of a chromophore at a given. Beer's law may be written simply as: In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Where a is absorbance (no units)
from slidetodoc.com
Beer's law may be written simply as: It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. Beer's law states that the absorbance is proportional to the concentration of the sample. Where a is absorbance (no units) The law is simply an application of the observation that, within certain ranges, the absorbance of a chromophore at a given. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is.
Spectrophotometry Key Concepts Lamberts Law of Absorption Beers
Beer Lambert Law Explained Beer's law states that the absorbance is proportional to the concentration of the sample. Where a is absorbance (no units) In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The law is simply an application of the observation that, within certain ranges, the absorbance of a chromophore at a given. Beer's law may be written simply as: Beer's law states that the absorbance is proportional to the concentration of the sample. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light.
From www.scienceabc.com
Beers Law Definition, History, Equation, Formula And Example Beer Lambert Law Explained In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Where a is absorbance (no units) It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. The law is simply an application of the observation that, within certain ranges, the absorbance of a chromophore at a. Beer Lambert Law Explained.
From www.youtube.com
General Application of Beerlambert law YouTube Beer Lambert Law Explained Beer's law states that the absorbance is proportional to the concentration of the sample. Where a is absorbance (no units) The law is simply an application of the observation that, within certain ranges, the absorbance of a chromophore at a given. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. Beer's. Beer Lambert Law Explained.
From www.edinst.com
Beer Lambert Law Transmittance & Absorbance Edinburgh Instruments Beer Lambert Law Explained Beer's law may be written simply as: Beer's law states that the absorbance is proportional to the concentration of the sample. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The law is simply an application of the observation that, within certain ranges, the absorbance of a chromophore at a given. It provides. Beer Lambert Law Explained.
From ceajxuqm.blob.core.windows.net
Beer Lambert Law Frq at Aaron Salinas blog Beer Lambert Law Explained In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. The law is simply an application of the observation that, within certain ranges, the absorbance of a chromophore at a given. Where a is absorbance (no. Beer Lambert Law Explained.
From www.adda247.com
Beer Lambert Law Equation Derivation, Formula, Examples Beer Lambert Law Explained Beer's law states that the absorbance is proportional to the concentration of the sample. Beer's law may be written simply as: Where a is absorbance (no units) The law is simply an application of the observation that, within certain ranges, the absorbance of a chromophore at a given. It provides a mathematical relationship between the substance’s concentration in a solution. Beer Lambert Law Explained.
From www.thoughtco.com
Beer's Law Definition and Equation Beer Lambert Law Explained Beer's law states that the absorbance is proportional to the concentration of the sample. The law is simply an application of the observation that, within certain ranges, the absorbance of a chromophore at a given. Beer's law may be written simply as: It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light.. Beer Lambert Law Explained.
From sciencenotes.org
Beer's Law Equation and Example Beer Lambert Law Explained Beer's law may be written simply as: In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The law is simply an application of the observation that, within certain ranges, the absorbance of a chromophore at a given. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to. Beer Lambert Law Explained.
From www.researchgate.net
Schematics demonstrating the original BeerLambert Law and Modified Beer Lambert Law Explained Beer's law may be written simply as: In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. Where a is absorbance (no units) Beer's law states that the absorbance is proportional to the concentration of the. Beer Lambert Law Explained.
From chemistrytalk.org
BeerLambert Law ChemTalk Beer Lambert Law Explained Beer's law may be written simply as: Where a is absorbance (no units) Beer's law states that the absorbance is proportional to the concentration of the sample. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. The law is simply an application of the observation that, within certain ranges, the absorbance. Beer Lambert Law Explained.
From www.researchgate.net
Schematics demonstrating the original BeerLambert Law and Modified Beer Lambert Law Explained The law is simply an application of the observation that, within certain ranges, the absorbance of a chromophore at a given. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. Beer's law states that the absorbance is proportional to the concentration of the sample. Beer's law may be written simply as:. Beer Lambert Law Explained.
From www.youtube.com
Beer Law Lambert Law Limiting Law Absorbance Transmittance Beer Lambert Law Explained The law is simply an application of the observation that, within certain ranges, the absorbance of a chromophore at a given. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. Where a is absorbance (no units) Beer's law may be written simply as: In most experiments, molar absorptivity (ε) and the. Beer Lambert Law Explained.
From www.slideshare.net
Beer lambert Law Beer Lambert Law Explained Beer's law states that the absorbance is proportional to the concentration of the sample. Where a is absorbance (no units) In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Beer's law may be written simply as: The law is simply an application of the observation that, within certain ranges, the absorbance of a. Beer Lambert Law Explained.
From www.youtube.com
Analytical Instrumentation Tutorial 2 Beer Lambert Law YouTube Beer Lambert Law Explained The law is simply an application of the observation that, within certain ranges, the absorbance of a chromophore at a given. Beer's law states that the absorbance is proportional to the concentration of the sample. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Beer's law may be written simply as: It provides. Beer Lambert Law Explained.
From www.slideshare.net
beer lamberts law, colorimetery, nephlometry and turbidimetry Beer Lambert Law Explained The law is simply an application of the observation that, within certain ranges, the absorbance of a chromophore at a given. Where a is absorbance (no units) In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb. Beer Lambert Law Explained.
From www.youtube.com
Introduction to UVVis Spectroscopy 03 BeerLambert Law YouTube Beer Lambert Law Explained Beer's law may be written simply as: Beer's law states that the absorbance is proportional to the concentration of the sample. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. The law is simply an application of the observation that, within certain ranges, the absorbance of a chromophore at a given.. Beer Lambert Law Explained.
From www.researchgate.net
1 a Schematic representation for BeerLambert law for the measurement Beer Lambert Law Explained Beer's law states that the absorbance is proportional to the concentration of the sample. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The law is simply an application of the observation that, within certain ranges, the absorbance of a chromophore at a given. Beer's law may be written simply as: Where a. Beer Lambert Law Explained.
From chemdictionary.org
BeerLambert Law History, Definition & Example Calculation Beer Lambert Law Explained It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. Beer's law may be written simply as: In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The law is simply an application of the observation that, within certain ranges, the absorbance of a chromophore at. Beer Lambert Law Explained.
From slidetodoc.com
Spectrophotometry Key Concepts Lamberts Law of Absorption Beers Beer Lambert Law Explained Beer's law states that the absorbance is proportional to the concentration of the sample. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. Beer's law may be written simply as: In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The law is simply an. Beer Lambert Law Explained.
From chemistrypubs.com
BeerLambert Law Equation, Derivation, & Uses Chemistrupubs Beer Lambert Law Explained It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. Where a is absorbance (no units) Beer's law states that the absorbance is proportional to the concentration of the sample. The law is simply an application of the observation that, within certain ranges, the absorbance of a chromophore at a given. Beer's. Beer Lambert Law Explained.
From www.youtube.com
Beer Lambert's Law, Absorbance & Transmittance Spectrophotometry Beer Lambert Law Explained In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. The law is simply an application of the observation that, within certain ranges, the absorbance of a chromophore at a given. Beer's law may be written. Beer Lambert Law Explained.
From www.youtube.com
Beer and Lambert Law Derivation YouTube Beer Lambert Law Explained Where a is absorbance (no units) The law is simply an application of the observation that, within certain ranges, the absorbance of a chromophore at a given. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a). Beer Lambert Law Explained.
From lukewarmtakes.net
Spectrophotometry and Beer's Law Lukewarm Takes Beer Lambert Law Explained Where a is absorbance (no units) The law is simply an application of the observation that, within certain ranges, the absorbance of a chromophore at a given. Beer's law may be written simply as: Beer's law states that the absorbance is proportional to the concentration of the sample. It provides a mathematical relationship between the substance’s concentration in a solution. Beer Lambert Law Explained.
From scienceinfo.com
BeerLambert Law Statement, Derivation, Applications, Limitations Beer Lambert Law Explained Beer's law may be written simply as: In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Where a is absorbance (no units) It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. The law is simply an application of the observation that, within certain ranges,. Beer Lambert Law Explained.
From www.youtube.com
Beer Lambert law in Hindi Beer Lambert Law explained Lambert beer Beer Lambert Law Explained Beer's law may be written simply as: Where a is absorbance (no units) The law is simply an application of the observation that, within certain ranges, the absorbance of a chromophore at a given. Beer's law states that the absorbance is proportional to the concentration of the sample. In most experiments, molar absorptivity (ε) and the length (b) are constant,. Beer Lambert Law Explained.
From lelandchemclub.weebly.com
Leland Chemistry Club Leland Chemistry Club News Beer Lambert Law Explained It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. Where a is absorbance (no units) Beer's law may be written simply as: Beer's law states that the absorbance is proportional to the concentration of the sample. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a). Beer Lambert Law Explained.
From www.researchgate.net
3. Illustration to Beerlambert's law. Download Scientific Diagram Beer Lambert Law Explained The law is simply an application of the observation that, within certain ranges, the absorbance of a chromophore at a given. Beer's law may be written simply as: Where a is absorbance (no units) It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. Beer's law states that the absorbance is proportional. Beer Lambert Law Explained.
From www.youtube.com
Spectrophotometric terms and BeerLambert Law YouTube Beer Lambert Law Explained Beer's law may be written simply as: Beer's law states that the absorbance is proportional to the concentration of the sample. The law is simply an application of the observation that, within certain ranges, the absorbance of a chromophore at a given. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. It provides. Beer Lambert Law Explained.
From www.youtube.com
Derivation of Beer Lambert Law YouTube Beer Lambert Law Explained It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The law is simply an application of the observation that, within certain ranges, the absorbance of a chromophore at a given. Beer's law states that the. Beer Lambert Law Explained.
From www.youtube.com
Beer Lambert Law simplest explanation animation YouTube Beer Lambert Law Explained Beer's law states that the absorbance is proportional to the concentration of the sample. Where a is absorbance (no units) It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. The law is simply an application of the observation that, within certain ranges, the absorbance of a chromophore at a given. In. Beer Lambert Law Explained.
From cevhwrun.blob.core.windows.net
Beer Lambert Law Relationship Between Absorbance And Concentration at Beer Lambert Law Explained Beer's law may be written simply as: The law is simply an application of the observation that, within certain ranges, the absorbance of a chromophore at a given. Beer's law states that the absorbance is proportional to the concentration of the sample. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light.. Beer Lambert Law Explained.
From www.youtube.com
Beer Lambert law derivation and usage YouTube Beer Lambert Law Explained Beer's law may be written simply as: Where a is absorbance (no units) In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. Beer's law states that the absorbance is proportional to the concentration of the. Beer Lambert Law Explained.
From joibnsohl.blob.core.windows.net
Spectrophotometry Learn The BeerLambert Law With Absorbance Beer Lambert Law Explained In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The law is simply an application of the observation that, within certain ranges, the absorbance of a chromophore at a given. Beer's law may be written simply as: Where a is absorbance (no units) Beer's law states that the absorbance is proportional to the. Beer Lambert Law Explained.
From ceyopxll.blob.core.windows.net
Beer Lambert Law Notes at Christina Wadsworth blog Beer Lambert Law Explained Beer's law may be written simply as: Beer's law states that the absorbance is proportional to the concentration of the sample. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. The law is simply an application of the observation that, within certain ranges, the absorbance of a chromophore at a given.. Beer Lambert Law Explained.
From www.youtube.com
Beer's Law Overview YouTube Beer Lambert Law Explained In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Beer's law states that the absorbance is proportional to the concentration of the sample. Beer's law may be written simply as: Where a is absorbance (no units) The law is simply an application of the observation that, within certain ranges, the absorbance of a. Beer Lambert Law Explained.
From www.youtube.com
BeerLambert law in easy way YouTube Beer Lambert Law Explained It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. Where a is absorbance (no units) The law is simply an application of the observation that, within certain ranges, the absorbance of a chromophore at a given. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a). Beer Lambert Law Explained.