Sources Of Error In Calorimeter . Use your calculated average deviation and relative error in your discussion. Apply the first law of thermodynamics to calorimetry. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. Any error analysis of a calorimetry experiment must take into consideration the flow of heat from system to calorimeter to other parts of the surroundings. (an average deviation of 0.02 j/g°c and a relative. Aside from possible sources of error, another limitation involves the kinds of reactions you can study. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. And any design of a. The biggest source of error in calorimetry is usually unwanted heat loss to the surroundings. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. This can be reduced by insulating the sides of the calorimeter and adding a lid. For example, when an exothermic reaction occurs in solution in a calorimeter, the.
from sincerenewsknight.blogspot.com
Apply the first law of thermodynamics to calorimetry. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. The biggest source of error in calorimetry is usually unwanted heat loss to the surroundings. (an average deviation of 0.02 j/g°c and a relative. For example, when an exothermic reaction occurs in solution in a calorimeter, the. Aside from possible sources of error, another limitation involves the kinds of reactions you can study. Any error analysis of a calorimetry experiment must take into consideration the flow of heat from system to calorimeter to other parts of the surroundings. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. And any design of a.
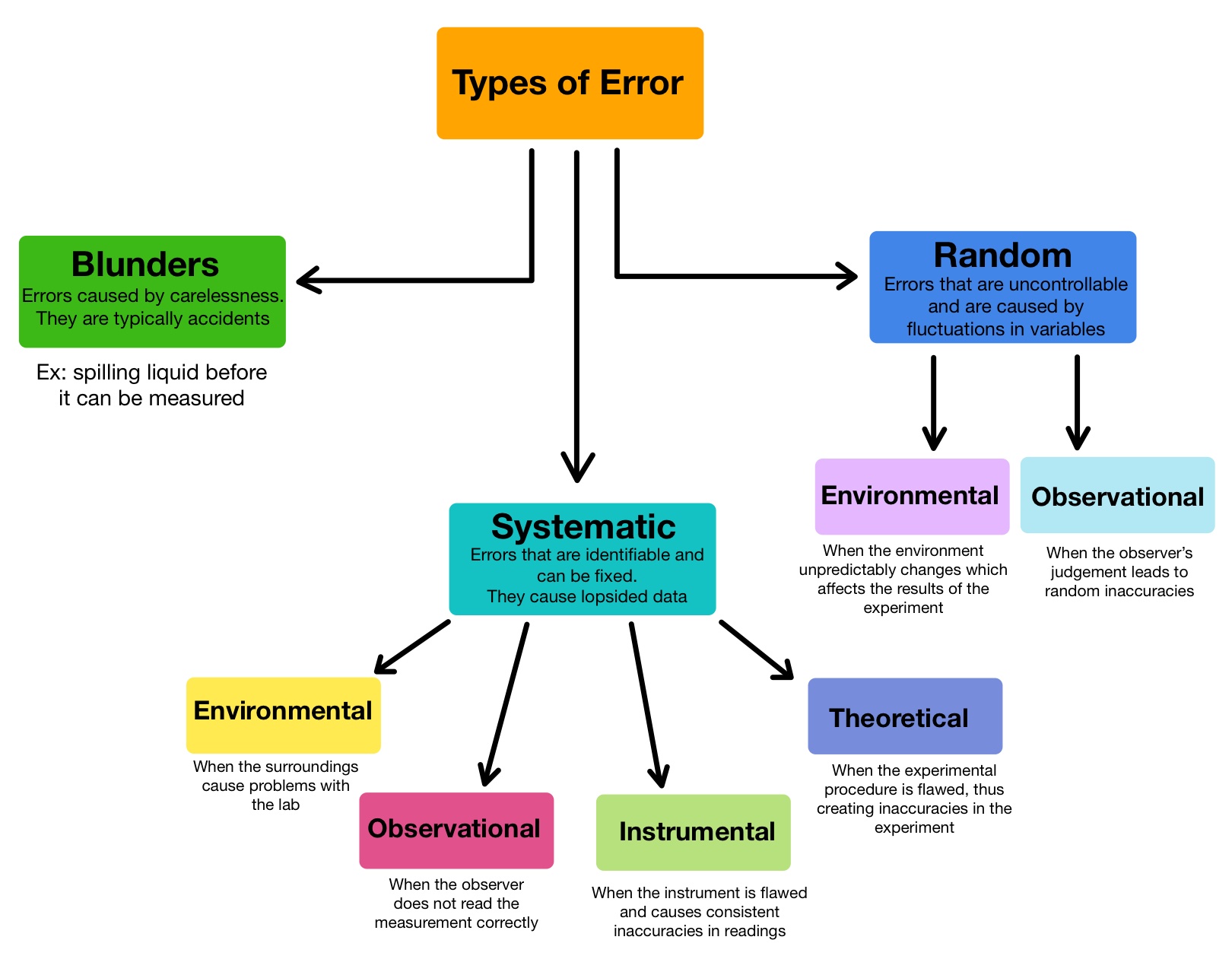
Types of Errors in Measurement
Sources Of Error In Calorimeter A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. And any design of a. Aside from possible sources of error, another limitation involves the kinds of reactions you can study. Use your calculated average deviation and relative error in your discussion. This can be reduced by insulating the sides of the calorimeter and adding a lid. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. (an average deviation of 0.02 j/g°c and a relative. For example, when an exothermic reaction occurs in solution in a calorimeter, the. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. The biggest source of error in calorimetry is usually unwanted heat loss to the surroundings. Any error analysis of a calorimetry experiment must take into consideration the flow of heat from system to calorimeter to other parts of the surroundings. Apply the first law of thermodynamics to calorimetry. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process.
From www.slideserve.com
PPT Chapter 14 Heat PowerPoint Presentation, free download ID6832402 Sources Of Error In Calorimeter A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Any error analysis of a calorimetry experiment must take into consideration the flow of heat from system to calorimeter to other parts of the surroundings. Use your calculated average deviation and relative error in your discussion. Apply the first law of. Sources Of Error In Calorimeter.
From www.slideserve.com
PPT Argon purity measurement of the calorimeter PowerPoint Presentation ID5882350 Sources Of Error In Calorimeter And any design of a. The biggest source of error in calorimetry is usually unwanted heat loss to the surroundings. For example, when an exothermic reaction occurs in solution in a calorimeter, the. Any error analysis of a calorimetry experiment must take into consideration the flow of heat from system to calorimeter to other parts of the surroundings. A calorimeter. Sources Of Error In Calorimeter.
From chem.libretexts.org
7.3 Heats of Reactions and Calorimetry Chemistry LibreTexts Sources Of Error In Calorimeter Apply the first law of thermodynamics to calorimetry. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. For example, when an exothermic. This can be reduced by insulating the sides of the calorimeter and adding a lid. (an average deviation of 0.02 j/g°c and a relative. A calorimeter is a device used to. Sources Of Error In Calorimeter.
From erwinnuza.blogspot.com
Types Of Experimental Errors Reasons for Error in a Chemistry Experiment Sciencing In any Sources Of Error In Calorimeter Use your calculated average deviation and relative error in your discussion. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. This can be reduced by insulating the sides of the calorimeter and adding a lid. And any design of a. Compare heat flow from hot. Sources Of Error In Calorimeter.
From sincerenewsknight.blogspot.com
Types of Errors in Measurement Sources Of Error In Calorimeter The biggest source of error in calorimetry is usually unwanted heat loss to the surroundings. Aside from possible sources of error, another limitation involves the kinds of reactions you can study. And any design of a. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. This can be reduced by. Sources Of Error In Calorimeter.
From www.researchgate.net
(PDF) A Source of Systematic Errors in the Determination of Critical Micelle Concentration and Sources Of Error In Calorimeter For example, when an exothermic reaction occurs in solution in a calorimeter, the. Any error analysis of a calorimetry experiment must take into consideration the flow of heat from system to calorimeter to other parts of the surroundings. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Use your calculated average deviation and. Sources Of Error In Calorimeter.
From www.scribd.com
Determining Specific Heat Capacity Through Calorimetry An Analysis of Heat Transfer, Thermal Sources Of Error In Calorimeter For example, when an exothermic reaction occurs in solution in a calorimeter, the. This can be reduced by insulating the sides of the calorimeter and adding a lid. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Any error analysis of a calorimetry experiment must take into consideration the flow. Sources Of Error In Calorimeter.
From studylib.net
5.1 b) CALORIMETRY Sources Of Error In Calorimeter Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. And any design of a. (an average deviation of 0.02 j/g°c and a relative. This can be reduced by insulating the sides of the calorimeter and adding a lid. For example, when an exothermic reaction occurs in solution in a calorimeter, the. The biggest. Sources Of Error In Calorimeter.
From www.youtube.com
Effects of experimental errors on enthalpy measurements YouTube Sources Of Error In Calorimeter The biggest source of error in calorimetry is usually unwanted heat loss to the surroundings. Aside from possible sources of error, another limitation involves the kinds of reactions you can study. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. This can be reduced by insulating the sides of the. Sources Of Error In Calorimeter.
From elsmar.com
Sources of Measurement Error Sources Of Error In Calorimeter Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Apply the first law of thermodynamics to calorimetry. And any design of a. The biggest source of error in calorimetry is usually unwanted heat loss to the surroundings. (an average deviation of 0.02 j/g°c and a relative. Any error analysis of a calorimetry experiment. Sources Of Error In Calorimeter.
From chem.libretexts.org
11.5 Reaction Calorimetry Chemistry LibreTexts Sources Of Error In Calorimeter The biggest source of error in calorimetry is usually unwanted heat loss to the surroundings. Aside from possible sources of error, another limitation involves the kinds of reactions you can study. For example, when an exothermic reaction occurs in solution in a calorimeter, the. Any error analysis of a calorimetry experiment must take into consideration the flow of heat from. Sources Of Error In Calorimeter.
From www.numerade.com
4. The calorimeter used in this experiment has no insulated lid. Is this a potential source of Sources Of Error In Calorimeter A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic reaction occurs in solution in a calorimeter, the. Any error analysis of a calorimetry experiment must take into consideration the flow of heat from system to calorimeter to other parts of the surroundings. Aside from possible. Sources Of Error In Calorimeter.
From www.education.com
Calorimetry Bomb Calorimeter Experiment Sources Of Error In Calorimeter (an average deviation of 0.02 j/g°c and a relative. This can be reduced by insulating the sides of the calorimeter and adding a lid. Use your calculated average deviation and relative error in your discussion. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Aside from possible sources of error,. Sources Of Error In Calorimeter.
From hxelhkhvw.blob.core.windows.net
How To Calculate Percent Error In Calorimetry at Smart blog Sources Of Error In Calorimeter (an average deviation of 0.02 j/g°c and a relative. Use your calculated average deviation and relative error in your discussion. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Any error analysis of a calorimetry experiment must take into consideration the flow of heat from system to calorimeter to other parts of the. Sources Of Error In Calorimeter.
From www.researchgate.net
Sources of error in calorimetry measurements Download Scientific Diagram Sources Of Error In Calorimeter A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. (an average deviation of 0.02 j/g°c and a relative. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. A calorimeter is a device used to measure the amount of. Sources Of Error In Calorimeter.
From www.thoughtco.com
Calorimeter Definition in Chemistry Sources Of Error In Calorimeter This can be reduced by insulating the sides of the calorimeter and adding a lid. Apply the first law of thermodynamics to calorimetry. For example, when an exothermic. The biggest source of error in calorimetry is usually unwanted heat loss to the surroundings. (an average deviation of 0.02 j/g°c and a relative. And any design of a. Use your calculated. Sources Of Error In Calorimeter.
From www.scribd.com
EXPERIMENT 302 Heat and Calorimetry Analysis Sources of Error PDF Heat Calorimetry Sources Of Error In Calorimeter Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Any error analysis of a calorimetry experiment must take into consideration the flow of heat from system to calorimeter to other parts of the surroundings. For example, when an exothermic reaction occurs in solution in a calorimeter, the. A calorimeter is a device used. Sources Of Error In Calorimeter.
From www.slideserve.com
PPT Heat of Combustion PowerPoint Presentation, free download ID6750144 Sources Of Error In Calorimeter A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. Aside from possible sources of error, another limitation involves the kinds of reactions you can study. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Use. Sources Of Error In Calorimeter.
From www.slideserve.com
PPT Differential Scanning Calorimetry PowerPoint Presentation, free download ID9512837 Sources Of Error In Calorimeter Apply the first law of thermodynamics to calorimetry. Any error analysis of a calorimetry experiment must take into consideration the flow of heat from system to calorimeter to other parts of the surroundings. This can be reduced by insulating the sides of the calorimeter and adding a lid. Compare heat flow from hot to cold objects in an ideal calorimeter. Sources Of Error In Calorimeter.
From github.com
GitHub saviss33/Calorimeter Temperature error associated in heat loss of calorimeter data Sources Of Error In Calorimeter Aside from possible sources of error, another limitation involves the kinds of reactions you can study. (an average deviation of 0.02 j/g°c and a relative. For example, when an exothermic reaction occurs in solution in a calorimeter, the. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Apply the first. Sources Of Error In Calorimeter.
From learningmagicriegel.z4.web.core.windows.net
Coffee Cup Calorimeter Calculator Sources Of Error In Calorimeter The biggest source of error in calorimetry is usually unwanted heat loss to the surroundings. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. This can be reduced by insulating the sides of the calorimeter and adding a lid. Any error analysis of a calorimetry experiment must take into consideration the flow of. Sources Of Error In Calorimeter.
From www.scribd.com
Error calorimeter constant PDF Temperature Heat Sources Of Error In Calorimeter This can be reduced by insulating the sides of the calorimeter and adding a lid. For example, when an exothermic reaction occurs in solution in a calorimeter, the. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. (an average deviation of 0.02 j/g°c and a. Sources Of Error In Calorimeter.
From www.numerade.com
SOLVED Name three possible sources of error in a calorimetry experiment. Sources Of Error In Calorimeter This can be reduced by insulating the sides of the calorimeter and adding a lid. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. And any design of a. Aside from possible sources of error, another limitation involves the kinds of reactions you can study.. Sources Of Error In Calorimeter.
From www.youtube.com
R1.1.4 What are the sources of error in calorimetry? YouTube Sources Of Error In Calorimeter Any error analysis of a calorimetry experiment must take into consideration the flow of heat from system to calorimeter to other parts of the surroundings. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. This can be reduced by insulating the sides of the calorimeter. Sources Of Error In Calorimeter.
From tukioka-clinic.com
😂 Soda can calorimeter sources of error. What Is a Calorimeter & What Are Its Limitations?. 2019 Sources Of Error In Calorimeter Use your calculated average deviation and relative error in your discussion. (an average deviation of 0.02 j/g°c and a relative. And any design of a. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. Compare heat flow from hot to cold objects in an ideal. Sources Of Error In Calorimeter.
From www.slideshare.net
4 Calorimetry Sources Of Error In Calorimeter And any design of a. The biggest source of error in calorimetry is usually unwanted heat loss to the surroundings. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Apply the first law of thermodynamics to calorimetry. For example, when an exothermic. A calorimeter is a device used to measure the amount of. Sources Of Error In Calorimeter.
From www.numerade.com
Name three possible sources of error in a calorimetry experiment. Numerade Sources Of Error In Calorimeter Any error analysis of a calorimetry experiment must take into consideration the flow of heat from system to calorimeter to other parts of the surroundings. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. And any design of a. A calorimeter is a device used to measure the amount of. Sources Of Error In Calorimeter.
From courses.lumenlearning.com
Calorimetry Chemistry Sources Of Error In Calorimeter And any design of a. Apply the first law of thermodynamics to calorimetry. Aside from possible sources of error, another limitation involves the kinds of reactions you can study. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Use your calculated average deviation and relative error in your discussion. The biggest source of. Sources Of Error In Calorimeter.
From www.slideshare.net
4 Calorimetry Sources Of Error In Calorimeter For example, when an exothermic. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Apply the first law of thermodynamics to calorimetry. (an average deviation of 0.02 j/g°c and a relative. Use your calculated. Sources Of Error In Calorimeter.
From www.numerade.com
SOLVED Method Error Calorimeter Heat loss from the calorimeter is always a source of error Sources Of Error In Calorimeter And any design of a. For example, when an exothermic reaction occurs in solution in a calorimeter, the. The biggest source of error in calorimetry is usually unwanted heat loss to the surroundings. Any error analysis of a calorimetry experiment must take into consideration the flow of heat from system to calorimeter to other parts of the surroundings. (an average. Sources Of Error In Calorimeter.
From www.learnable.education
Year 11 Chemistry Practical Investigation Calorimetry Experiment Sources Of Error In Calorimeter A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Any error analysis of a calorimetry experiment must take into consideration the flow of heat from system to calorimeter to other parts of the surroundings. This can be reduced by insulating the sides of the calorimeter and adding a lid. Use. Sources Of Error In Calorimeter.
From www.numerade.com
SOLVED Method Error Calorimeter Heat loss from the calorimeter is always a source of error Sources Of Error In Calorimeter Any error analysis of a calorimetry experiment must take into consideration the flow of heat from system to calorimeter to other parts of the surroundings. Use your calculated average deviation and relative error in your discussion. The biggest source of error in calorimetry is usually unwanted heat loss to the surroundings. For example, when an exothermic reaction occurs in solution. Sources Of Error In Calorimeter.
From www.ilectureonline.com
Sources Of Error In Calorimeter Any error analysis of a calorimetry experiment must take into consideration the flow of heat from system to calorimeter to other parts of the surroundings. (an average deviation of 0.02 j/g°c and a relative. For example, when an exothermic. Use your calculated average deviation and relative error in your discussion. A calorimeter is a device used to measure the amount. Sources Of Error In Calorimeter.
From www.chegg.com
Bomb Calorimetry CHEM 3625 Prelab Section 1 When a Sources Of Error In Calorimeter The biggest source of error in calorimetry is usually unwanted heat loss to the surroundings. And any design of a. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Aside from possible sources of error, another limitation involves the kinds of reactions you can study. A calorimeter is a device used to measure. Sources Of Error In Calorimeter.
From www.numerade.com
SOLVED One of the common sources of error in the determination of the specific heat capacity of Sources Of Error In Calorimeter This can be reduced by insulating the sides of the calorimeter and adding a lid. The biggest source of error in calorimetry is usually unwanted heat loss to the surroundings. (an average deviation of 0.02 j/g°c and a relative. And any design of a. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter.. Sources Of Error In Calorimeter.