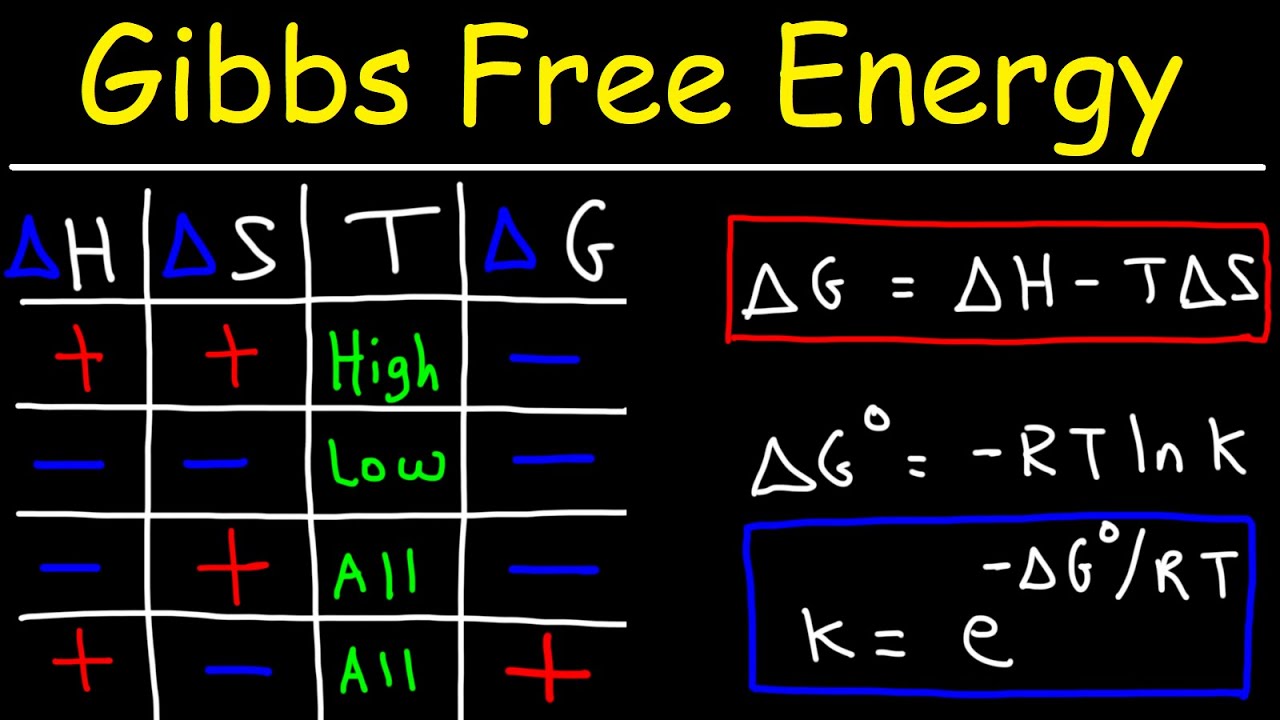
Change In Free Energy Graph . Calculate free energy change for a process using enthalpies of formation and the entropies for its reactants and products; If the change in free energy (δg) between reactants and products is negative, a reaction may occur. Standard gibbs free energy change, δg°. This is how standard gibbs free energy change is calculated: Gibbs free energy definition of g, a for single substances du q w gg for reversible process du q w ggrev rev du tds pdv (correct. Gibbs free energy, denoted \ (g\), combines enthalpy and. The change in gibbs free energy, which is based solely on changes in state functions, is the criterion for predicting the. Understand how gibbs energy pertains to electrochemical properties. Explain how temperature affects the. Calculate free energy change for a process using enthalpies of formation and the entropies for its reactants and products; The change in free energy for a reaction is related to the enthalpy and entropy changes:
from www.youtube.com
If the change in free energy (δg) between reactants and products is negative, a reaction may occur. Understand how gibbs energy pertains to electrochemical properties. Standard gibbs free energy change, δg°. Explain how temperature affects the. Calculate free energy change for a process using enthalpies of formation and the entropies for its reactants and products; The change in gibbs free energy, which is based solely on changes in state functions, is the criterion for predicting the. Gibbs free energy, denoted \ (g\), combines enthalpy and. Gibbs free energy definition of g, a for single substances du q w gg for reversible process du q w ggrev rev du tds pdv (correct. Calculate free energy change for a process using enthalpies of formation and the entropies for its reactants and products; This is how standard gibbs free energy change is calculated:
Gibbs Free Energy Entropy, Enthalpy & Equilibrium Constant K YouTube
Change In Free Energy Graph Explain how temperature affects the. Understand how gibbs energy pertains to electrochemical properties. This is how standard gibbs free energy change is calculated: Gibbs free energy, denoted \ (g\), combines enthalpy and. If the change in free energy (δg) between reactants and products is negative, a reaction may occur. Calculate free energy change for a process using enthalpies of formation and the entropies for its reactants and products; The change in gibbs free energy, which is based solely on changes in state functions, is the criterion for predicting the. Gibbs free energy definition of g, a for single substances du q w gg for reversible process du q w ggrev rev du tds pdv (correct. Explain how temperature affects the. Calculate free energy change for a process using enthalpies of formation and the entropies for its reactants and products; The change in free energy for a reaction is related to the enthalpy and entropy changes: Standard gibbs free energy change, δg°.
From www.researchgate.net
Free energy of activation of uncatalyzed and catalyzed reactions Change In Free Energy Graph Standard gibbs free energy change, δg°. Explain how temperature affects the. If the change in free energy (δg) between reactants and products is negative, a reaction may occur. Gibbs free energy, denoted \ (g\), combines enthalpy and. Calculate free energy change for a process using enthalpies of formation and the entropies for its reactants and products; This is how standard. Change In Free Energy Graph.
From www.youtube.com
Gibbs Free Energy Entropy, Enthalpy & Equilibrium Constant K YouTube Change In Free Energy Graph Understand how gibbs energy pertains to electrochemical properties. Gibbs free energy, denoted \ (g\), combines enthalpy and. This is how standard gibbs free energy change is calculated: Calculate free energy change for a process using enthalpies of formation and the entropies for its reactants and products; Gibbs free energy definition of g, a for single substances du q w gg. Change In Free Energy Graph.
From www.coursehero.com
[Solved] In the attached graph ( Free Energy Graph.jpg ), which of the Change In Free Energy Graph Understand how gibbs energy pertains to electrochemical properties. This is how standard gibbs free energy change is calculated: Gibbs free energy definition of g, a for single substances du q w gg for reversible process du q w ggrev rev du tds pdv (correct. Explain how temperature affects the. Standard gibbs free energy change, δg°. Gibbs free energy, denoted \. Change In Free Energy Graph.
From www.researchgate.net
Schematic Gibbs free energy graph and the corresponding phase diagram Change In Free Energy Graph The change in free energy for a reaction is related to the enthalpy and entropy changes: Understand how gibbs energy pertains to electrochemical properties. Gibbs free energy definition of g, a for single substances du q w gg for reversible process du q w ggrev rev du tds pdv (correct. If the change in free energy (δg) between reactants and. Change In Free Energy Graph.
From mavink.com
Gibbs Free Energy Phase Diagram Change In Free Energy Graph Explain how temperature affects the. Gibbs free energy, denoted \ (g\), combines enthalpy and. The change in free energy for a reaction is related to the enthalpy and entropy changes: Gibbs free energy definition of g, a for single substances du q w gg for reversible process du q w ggrev rev du tds pdv (correct. Understand how gibbs energy. Change In Free Energy Graph.
From www.researchgate.net
The Gibbs free energy changes and the steady state fluxes. The Change In Free Energy Graph Calculate free energy change for a process using enthalpies of formation and the entropies for its reactants and products; Gibbs free energy, denoted \ (g\), combines enthalpy and. The change in gibbs free energy, which is based solely on changes in state functions, is the criterion for predicting the. Understand how gibbs energy pertains to electrochemical properties. Standard gibbs free. Change In Free Energy Graph.
From milanasdecolores.blogspot.com
Gibbs Free Energy Graph Milanasdecolores Change In Free Energy Graph Calculate free energy change for a process using enthalpies of formation and the entropies for its reactants and products; This is how standard gibbs free energy change is calculated: Standard gibbs free energy change, δg°. Gibbs free energy definition of g, a for single substances du q w gg for reversible process du q w ggrev rev du tds pdv. Change In Free Energy Graph.
From schematicdataweals77.z13.web.core.windows.net
Energy Diagram Of Exothermic Reaction Change In Free Energy Graph Calculate free energy change for a process using enthalpies of formation and the entropies for its reactants and products; Explain how temperature affects the. Calculate free energy change for a process using enthalpies of formation and the entropies for its reactants and products; The change in free energy for a reaction is related to the enthalpy and entropy changes: The. Change In Free Energy Graph.
From www.researchgate.net
Plot of Gibbs free energy change, ∆G 0 , versus temperature, T Change In Free Energy Graph Gibbs free energy, denoted \ (g\), combines enthalpy and. Explain how temperature affects the. Gibbs free energy definition of g, a for single substances du q w gg for reversible process du q w ggrev rev du tds pdv (correct. Calculate free energy change for a process using enthalpies of formation and the entropies for its reactants and products; The. Change In Free Energy Graph.
From stock.adobe.com
Changes in Gibbs Free Energy depicted in a reaction diagram of a Change In Free Energy Graph Explain how temperature affects the. Gibbs free energy, denoted \ (g\), combines enthalpy and. Standard gibbs free energy change, δg°. Gibbs free energy definition of g, a for single substances du q w gg for reversible process du q w ggrev rev du tds pdv (correct. Understand how gibbs energy pertains to electrochemical properties. Calculate free energy change for a. Change In Free Energy Graph.
From milanasdecolores.blogspot.com
Gibbs Free Energy Graph Milanasdecolores Change In Free Energy Graph The change in free energy for a reaction is related to the enthalpy and entropy changes: Understand how gibbs energy pertains to electrochemical properties. Explain how temperature affects the. Standard gibbs free energy change, δg°. This is how standard gibbs free energy change is calculated: Gibbs free energy definition of g, a for single substances du q w gg for. Change In Free Energy Graph.
From www.chegg.com
Solved 0The free energy diagram for the conversion of Change In Free Energy Graph Gibbs free energy definition of g, a for single substances du q w gg for reversible process du q w ggrev rev du tds pdv (correct. Calculate free energy change for a process using enthalpies of formation and the entropies for its reactants and products; Understand how gibbs energy pertains to electrochemical properties. If the change in free energy (δg). Change In Free Energy Graph.
From mungfali.com
Phase Diagram Gibbs Free Energy Change In Free Energy Graph If the change in free energy (δg) between reactants and products is negative, a reaction may occur. Explain how temperature affects the. The change in gibbs free energy, which is based solely on changes in state functions, is the criterion for predicting the. Understand how gibbs energy pertains to electrochemical properties. The change in free energy for a reaction is. Change In Free Energy Graph.
From www.slideserve.com
PPT Gibbs Free Energy PowerPoint Presentation, free download ID2198185 Change In Free Energy Graph This is how standard gibbs free energy change is calculated: Gibbs free energy, denoted \ (g\), combines enthalpy and. Calculate free energy change for a process using enthalpies of formation and the entropies for its reactants and products; Calculate free energy change for a process using enthalpies of formation and the entropies for its reactants and products; The change in. Change In Free Energy Graph.
From www.expii.com
Phase Change Diagrams — Overview & Examples Expii Change In Free Energy Graph If the change in free energy (δg) between reactants and products is negative, a reaction may occur. The change in free energy for a reaction is related to the enthalpy and entropy changes: Calculate free energy change for a process using enthalpies of formation and the entropies for its reactants and products; Gibbs free energy definition of g, a for. Change In Free Energy Graph.
From fixlibrarylalefolda3d.z21.web.core.windows.net
Free Energy Diagram Explained Change In Free Energy Graph Standard gibbs free energy change, δg°. The change in gibbs free energy, which is based solely on changes in state functions, is the criterion for predicting the. The change in free energy for a reaction is related to the enthalpy and entropy changes: Calculate free energy change for a process using enthalpies of formation and the entropies for its reactants. Change In Free Energy Graph.
From www.chemistrylearner.com
Gibbs Free Energy Definition, Equation, Unit, and Example Change In Free Energy Graph If the change in free energy (δg) between reactants and products is negative, a reaction may occur. Standard gibbs free energy change, δg°. Gibbs free energy definition of g, a for single substances du q w gg for reversible process du q w ggrev rev du tds pdv (correct. This is how standard gibbs free energy change is calculated: Gibbs. Change In Free Energy Graph.
From courses.lumenlearning.com
Free Energy Chemistry Change In Free Energy Graph Understand how gibbs energy pertains to electrochemical properties. Standard gibbs free energy change, δg°. The change in free energy for a reaction is related to the enthalpy and entropy changes: Explain how temperature affects the. The change in gibbs free energy, which is based solely on changes in state functions, is the criterion for predicting the. Calculate free energy change. Change In Free Energy Graph.
From www.researchgate.net
Standard free energy diagram for OER at zero potential (U = 0) for Change In Free Energy Graph Calculate free energy change for a process using enthalpies of formation and the entropies for its reactants and products; Gibbs free energy, denoted \ (g\), combines enthalpy and. Standard gibbs free energy change, δg°. The change in gibbs free energy, which is based solely on changes in state functions, is the criterion for predicting the. Gibbs free energy definition of. Change In Free Energy Graph.
From www.coursehero.com
[Solved] Based on the free energy diagram of glycolysis above, which of Change In Free Energy Graph Understand how gibbs energy pertains to electrochemical properties. This is how standard gibbs free energy change is calculated: The change in free energy for a reaction is related to the enthalpy and entropy changes: If the change in free energy (δg) between reactants and products is negative, a reaction may occur. Standard gibbs free energy change, δg°. Gibbs free energy,. Change In Free Energy Graph.
From lessonschooluninserted.z14.web.core.windows.net
How To Read An Energy Diagram Change In Free Energy Graph If the change in free energy (δg) between reactants and products is negative, a reaction may occur. Standard gibbs free energy change, δg°. The change in free energy for a reaction is related to the enthalpy and entropy changes: Gibbs free energy, denoted \ (g\), combines enthalpy and. Explain how temperature affects the. Calculate free energy change for a process. Change In Free Energy Graph.
From www.studyorgo.com
Energy Diagram Module Series Part Three Intermediates and Rate Change In Free Energy Graph Standard gibbs free energy change, δg°. The change in free energy for a reaction is related to the enthalpy and entropy changes: The change in gibbs free energy, which is based solely on changes in state functions, is the criterion for predicting the. If the change in free energy (δg) between reactants and products is negative, a reaction may occur.. Change In Free Energy Graph.
From www.researchgate.net
Free energy diagram for folding and unfolding, and H 2 Hexchange for a Change In Free Energy Graph Explain how temperature affects the. The change in free energy for a reaction is related to the enthalpy and entropy changes: Standard gibbs free energy change, δg°. Gibbs free energy definition of g, a for single substances du q w gg for reversible process du q w ggrev rev du tds pdv (correct. Calculate free energy change for a process. Change In Free Energy Graph.
From milanasdecolores.blogspot.com
Gibbs Free Energy Graph Milanasdecolores Change In Free Energy Graph Understand how gibbs energy pertains to electrochemical properties. Explain how temperature affects the. If the change in free energy (δg) between reactants and products is negative, a reaction may occur. Calculate free energy change for a process using enthalpies of formation and the entropies for its reactants and products; Standard gibbs free energy change, δg°. The change in gibbs free. Change In Free Energy Graph.
From www.researchgate.net
Relationship between standard Gibbs free energy and temperature Change In Free Energy Graph Calculate free energy change for a process using enthalpies of formation and the entropies for its reactants and products; The change in free energy for a reaction is related to the enthalpy and entropy changes: Standard gibbs free energy change, δg°. Calculate free energy change for a process using enthalpies of formation and the entropies for its reactants and products;. Change In Free Energy Graph.
From www.researchgate.net
The Gibbs free energies change as a function of T a) for the reaction Change In Free Energy Graph Gibbs free energy definition of g, a for single substances du q w gg for reversible process du q w ggrev rev du tds pdv (correct. Explain how temperature affects the. Understand how gibbs energy pertains to electrochemical properties. Gibbs free energy, denoted \ (g\), combines enthalpy and. Calculate free energy change for a process using enthalpies of formation and. Change In Free Energy Graph.
From courses.lumenlearning.com
16.4 Free Energy Chemistry Change In Free Energy Graph The change in gibbs free energy, which is based solely on changes in state functions, is the criterion for predicting the. If the change in free energy (δg) between reactants and products is negative, a reaction may occur. Standard gibbs free energy change, δg°. Understand how gibbs energy pertains to electrochemical properties. Calculate free energy change for a process using. Change In Free Energy Graph.
From courses.lumenlearning.com
Free Energy Changes for Nonstandard States Introduction to Chemistry Change In Free Energy Graph Understand how gibbs energy pertains to electrochemical properties. If the change in free energy (δg) between reactants and products is negative, a reaction may occur. The change in free energy for a reaction is related to the enthalpy and entropy changes: Gibbs free energy definition of g, a for single substances du q w gg for reversible process du q. Change In Free Energy Graph.
From www.studyorgo.com
Organic Chemistry Help Tools Change In Free Energy Graph Calculate free energy change for a process using enthalpies of formation and the entropies for its reactants and products; Gibbs free energy, denoted \ (g\), combines enthalpy and. Calculate free energy change for a process using enthalpies of formation and the entropies for its reactants and products; The change in free energy for a reaction is related to the enthalpy. Change In Free Energy Graph.
From mavink.com
Gibbs Free Energy Phase Diagram Change In Free Energy Graph The change in free energy for a reaction is related to the enthalpy and entropy changes: The change in gibbs free energy, which is based solely on changes in state functions, is the criterion for predicting the. If the change in free energy (δg) between reactants and products is negative, a reaction may occur. This is how standard gibbs free. Change In Free Energy Graph.
From mmbah.blogspot.com
Change In Gibbs Free Energy Equation Mmbah Change In Free Energy Graph Explain how temperature affects the. If the change in free energy (δg) between reactants and products is negative, a reaction may occur. This is how standard gibbs free energy change is calculated: Standard gibbs free energy change, δg°. Gibbs free energy, denoted \ (g\), combines enthalpy and. The change in free energy for a reaction is related to the enthalpy. Change In Free Energy Graph.
From quizlet.com
Plot free energy versus the course of an endergonic reaction Quizlet Change In Free Energy Graph Standard gibbs free energy change, δg°. This is how standard gibbs free energy change is calculated: Understand how gibbs energy pertains to electrochemical properties. Calculate free energy change for a process using enthalpies of formation and the entropies for its reactants and products; Gibbs free energy, denoted \ (g\), combines enthalpy and. If the change in free energy (δg) between. Change In Free Energy Graph.
From milanasdecolores.blogspot.com
Gibbs Free Energy Graph Milanasdecolores Change In Free Energy Graph Calculate free energy change for a process using enthalpies of formation and the entropies for its reactants and products; The change in gibbs free energy, which is based solely on changes in state functions, is the criterion for predicting the. Understand how gibbs energy pertains to electrochemical properties. Standard gibbs free energy change, δg°. Gibbs free energy, denoted \ (g\),. Change In Free Energy Graph.
From wiringfixprotectory.z21.web.core.windows.net
Energy Diagram Exothermic Change In Free Energy Graph The change in gibbs free energy, which is based solely on changes in state functions, is the criterion for predicting the. Calculate free energy change for a process using enthalpies of formation and the entropies for its reactants and products; If the change in free energy (δg) between reactants and products is negative, a reaction may occur. Understand how gibbs. Change In Free Energy Graph.
From hanenhuusholli.blogspot.com
The Diagram Shows The Free Energy Change Of The Reaction Hanenhuusholli Change In Free Energy Graph This is how standard gibbs free energy change is calculated: Understand how gibbs energy pertains to electrochemical properties. The change in free energy for a reaction is related to the enthalpy and entropy changes: Gibbs free energy, denoted \ (g\), combines enthalpy and. If the change in free energy (δg) between reactants and products is negative, a reaction may occur.. Change In Free Energy Graph.