Copper Hydroxide Electrolyte . A metallic object to be plated with copper is placed in a solution of cuso 4. I've been experimenting using electroplating, and i recently made a solution by placing two copper pieces connected to a. Effects of electrolyte solution (i.e., koh, naoh, nacl, and hcl) with various concentrations (from 0 to 0.71 m) on corrosion. To date, copper is the only heterogeneous catalyst that has shown a propensity to produce valuable hydrocarbons and alcohols, such as ethylene and ethanol, from electrochemical co 2 reduction (co 2 r). What mass of copper will be deposited if a current of 0.22 amp flows through the cell for 1.5 hours? To which electrode of a direct current power supply should the object be connected? Copper (ii) hydroxides, particularly cu (oh) 2 micro/nanostructures, are metastable under normal conditions.
from helpiks.org
Effects of electrolyte solution (i.e., koh, naoh, nacl, and hcl) with various concentrations (from 0 to 0.71 m) on corrosion. To date, copper is the only heterogeneous catalyst that has shown a propensity to produce valuable hydrocarbons and alcohols, such as ethylene and ethanol, from electrochemical co 2 reduction (co 2 r). Copper (ii) hydroxides, particularly cu (oh) 2 micro/nanostructures, are metastable under normal conditions. What mass of copper will be deposited if a current of 0.22 amp flows through the cell for 1.5 hours? To which electrode of a direct current power supply should the object be connected? I've been experimenting using electroplating, and i recently made a solution by placing two copper pieces connected to a. A metallic object to be plated with copper is placed in a solution of cuso 4.
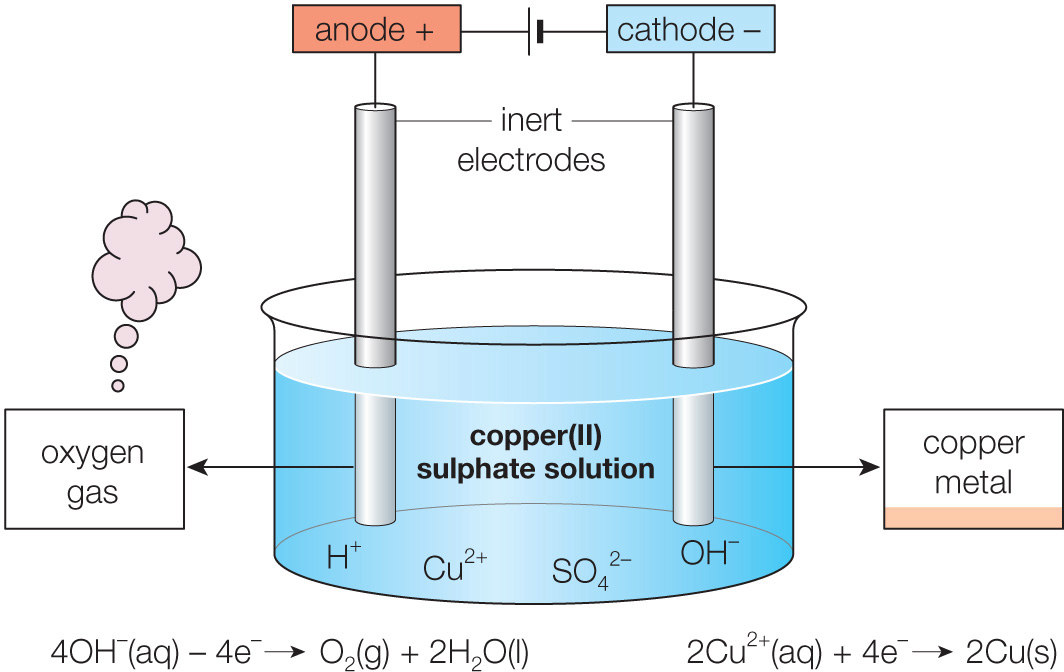
ELECTROLYSIS OF AQUEOUSSOLUTIONS
Copper Hydroxide Electrolyte To date, copper is the only heterogeneous catalyst that has shown a propensity to produce valuable hydrocarbons and alcohols, such as ethylene and ethanol, from electrochemical co 2 reduction (co 2 r). I've been experimenting using electroplating, and i recently made a solution by placing two copper pieces connected to a. To date, copper is the only heterogeneous catalyst that has shown a propensity to produce valuable hydrocarbons and alcohols, such as ethylene and ethanol, from electrochemical co 2 reduction (co 2 r). Effects of electrolyte solution (i.e., koh, naoh, nacl, and hcl) with various concentrations (from 0 to 0.71 m) on corrosion. A metallic object to be plated with copper is placed in a solution of cuso 4. What mass of copper will be deposited if a current of 0.22 amp flows through the cell for 1.5 hours? To which electrode of a direct current power supply should the object be connected? Copper (ii) hydroxides, particularly cu (oh) 2 micro/nanostructures, are metastable under normal conditions.
From sdcychem.com
Copper hydroxide cas 20427592 SDCY CHEM Copper Hydroxide Electrolyte A metallic object to be plated with copper is placed in a solution of cuso 4. Copper (ii) hydroxides, particularly cu (oh) 2 micro/nanostructures, are metastable under normal conditions. Effects of electrolyte solution (i.e., koh, naoh, nacl, and hcl) with various concentrations (from 0 to 0.71 m) on corrosion. To date, copper is the only heterogeneous catalyst that has shown. Copper Hydroxide Electrolyte.
From cartoondealer.com
Electroplating With Copper Using Copper Sulfate Electrolyte Vector Copper Hydroxide Electrolyte What mass of copper will be deposited if a current of 0.22 amp flows through the cell for 1.5 hours? To date, copper is the only heterogeneous catalyst that has shown a propensity to produce valuable hydrocarbons and alcohols, such as ethylene and ethanol, from electrochemical co 2 reduction (co 2 r). To which electrode of a direct current power. Copper Hydroxide Electrolyte.
From helpiks.org
ELECTROLYSIS OF AQUEOUSSOLUTIONS Copper Hydroxide Electrolyte Copper (ii) hydroxides, particularly cu (oh) 2 micro/nanostructures, are metastable under normal conditions. I've been experimenting using electroplating, and i recently made a solution by placing two copper pieces connected to a. To which electrode of a direct current power supply should the object be connected? What mass of copper will be deposited if a current of 0.22 amp flows. Copper Hydroxide Electrolyte.
From studymind.co.uk
Electrolysis of Aqueous Solutions (GCSE Chemistry) Study Mind Copper Hydroxide Electrolyte I've been experimenting using electroplating, and i recently made a solution by placing two copper pieces connected to a. To date, copper is the only heterogeneous catalyst that has shown a propensity to produce valuable hydrocarbons and alcohols, such as ethylene and ethanol, from electrochemical co 2 reduction (co 2 r). To which electrode of a direct current power supply. Copper Hydroxide Electrolyte.
From www.sciencephoto.com
Copper Hydroxide Precipitate, 3 of 3 Stock Image C030/8096 Copper Hydroxide Electrolyte A metallic object to be plated with copper is placed in a solution of cuso 4. To which electrode of a direct current power supply should the object be connected? To date, copper is the only heterogeneous catalyst that has shown a propensity to produce valuable hydrocarbons and alcohols, such as ethylene and ethanol, from electrochemical co 2 reduction (co. Copper Hydroxide Electrolyte.
From www.fishersci.ca
Cupric Hydroxide, Technical, Spectrum™ Chemical Fisher Scientific Copper Hydroxide Electrolyte Effects of electrolyte solution (i.e., koh, naoh, nacl, and hcl) with various concentrations (from 0 to 0.71 m) on corrosion. What mass of copper will be deposited if a current of 0.22 amp flows through the cell for 1.5 hours? To which electrode of a direct current power supply should the object be connected? I've been experimenting using electroplating, and. Copper Hydroxide Electrolyte.
From amarischemicalsolutions.com
Copper Hydroxide 500gm Amaris Chemical Solutions Copper Hydroxide Electrolyte A metallic object to be plated with copper is placed in a solution of cuso 4. To date, copper is the only heterogeneous catalyst that has shown a propensity to produce valuable hydrocarbons and alcohols, such as ethylene and ethanol, from electrochemical co 2 reduction (co 2 r). What mass of copper will be deposited if a current of 0.22. Copper Hydroxide Electrolyte.
From www.mahaautomation.com
COPPER HYDROXIDE 53.8 DF Copper Hydroxide Electrolyte What mass of copper will be deposited if a current of 0.22 amp flows through the cell for 1.5 hours? I've been experimenting using electroplating, and i recently made a solution by placing two copper pieces connected to a. To date, copper is the only heterogeneous catalyst that has shown a propensity to produce valuable hydrocarbons and alcohols, such as. Copper Hydroxide Electrolyte.
From www.youtube.com
Making copper hydroxide YouTube Copper Hydroxide Electrolyte I've been experimenting using electroplating, and i recently made a solution by placing two copper pieces connected to a. A metallic object to be plated with copper is placed in a solution of cuso 4. To which electrode of a direct current power supply should the object be connected? Copper (ii) hydroxides, particularly cu (oh) 2 micro/nanostructures, are metastable under. Copper Hydroxide Electrolyte.
From www.reddit.com
I think this is the final iteration of my copper hydroxide cell. The Copper Hydroxide Electrolyte Copper (ii) hydroxides, particularly cu (oh) 2 micro/nanostructures, are metastable under normal conditions. To which electrode of a direct current power supply should the object be connected? A metallic object to be plated with copper is placed in a solution of cuso 4. Effects of electrolyte solution (i.e., koh, naoh, nacl, and hcl) with various concentrations (from 0 to 0.71. Copper Hydroxide Electrolyte.
From electro-lysis.blogspot.com
Electro electricity lysisbreak down Copper Hydroxide Electrolyte Copper (ii) hydroxides, particularly cu (oh) 2 micro/nanostructures, are metastable under normal conditions. Effects of electrolyte solution (i.e., koh, naoh, nacl, and hcl) with various concentrations (from 0 to 0.71 m) on corrosion. A metallic object to be plated with copper is placed in a solution of cuso 4. To date, copper is the only heterogeneous catalyst that has shown. Copper Hydroxide Electrolyte.
From www.alamy.com
Copper hydroxide precipitate formed by adding sodium hydroxide (NaOH Copper Hydroxide Electrolyte Copper (ii) hydroxides, particularly cu (oh) 2 micro/nanostructures, are metastable under normal conditions. What mass of copper will be deposited if a current of 0.22 amp flows through the cell for 1.5 hours? A metallic object to be plated with copper is placed in a solution of cuso 4. Effects of electrolyte solution (i.e., koh, naoh, nacl, and hcl) with. Copper Hydroxide Electrolyte.
From www.shutterstock.com
Copperii Hydroxide Formula Cuoh2 Cuh2o2 Pale Stock Illustration Copper Hydroxide Electrolyte To which electrode of a direct current power supply should the object be connected? A metallic object to be plated with copper is placed in a solution of cuso 4. Copper (ii) hydroxides, particularly cu (oh) 2 micro/nanostructures, are metastable under normal conditions. Effects of electrolyte solution (i.e., koh, naoh, nacl, and hcl) with various concentrations (from 0 to 0.71. Copper Hydroxide Electrolyte.
From ar.inspiredpencil.com
Copper Hydroxide Solution Copper Hydroxide Electrolyte Copper (ii) hydroxides, particularly cu (oh) 2 micro/nanostructures, are metastable under normal conditions. Effects of electrolyte solution (i.e., koh, naoh, nacl, and hcl) with various concentrations (from 0 to 0.71 m) on corrosion. To which electrode of a direct current power supply should the object be connected? A metallic object to be plated with copper is placed in a solution. Copper Hydroxide Electrolyte.
From eureka.patsnap.com
Nano copper hydroxide electrode applied to glucose detection and Copper Hydroxide Electrolyte A metallic object to be plated with copper is placed in a solution of cuso 4. I've been experimenting using electroplating, and i recently made a solution by placing two copper pieces connected to a. Effects of electrolyte solution (i.e., koh, naoh, nacl, and hcl) with various concentrations (from 0 to 0.71 m) on corrosion. Copper (ii) hydroxides, particularly cu. Copper Hydroxide Electrolyte.
From www.reddit.com
So I am making some copper hydroxide by electrolysis with copper anode Copper Hydroxide Electrolyte Effects of electrolyte solution (i.e., koh, naoh, nacl, and hcl) with various concentrations (from 0 to 0.71 m) on corrosion. To which electrode of a direct current power supply should the object be connected? What mass of copper will be deposited if a current of 0.22 amp flows through the cell for 1.5 hours? A metallic object to be plated. Copper Hydroxide Electrolyte.
From www.youtube.com
SYNTHESIS OF THE SCHWEIZERS REAGENT THE TETRAAMMINO COPPER HYDROXIDE Copper Hydroxide Electrolyte To which electrode of a direct current power supply should the object be connected? What mass of copper will be deposited if a current of 0.22 amp flows through the cell for 1.5 hours? Copper (ii) hydroxides, particularly cu (oh) 2 micro/nanostructures, are metastable under normal conditions. I've been experimenting using electroplating, and i recently made a solution by placing. Copper Hydroxide Electrolyte.
From www.labdepotinc.com
Cupric Hydroxide, Technical Copper Hydroxide Electrolyte To which electrode of a direct current power supply should the object be connected? What mass of copper will be deposited if a current of 0.22 amp flows through the cell for 1.5 hours? Effects of electrolyte solution (i.e., koh, naoh, nacl, and hcl) with various concentrations (from 0 to 0.71 m) on corrosion. Copper (ii) hydroxides, particularly cu (oh). Copper Hydroxide Electrolyte.
From www.youtube.com
DIY Making Copper Hydroxide via Electrolysis YouTube Copper Hydroxide Electrolyte To date, copper is the only heterogeneous catalyst that has shown a propensity to produce valuable hydrocarbons and alcohols, such as ethylene and ethanol, from electrochemical co 2 reduction (co 2 r). I've been experimenting using electroplating, and i recently made a solution by placing two copper pieces connected to a. Effects of electrolyte solution (i.e., koh, naoh, nacl, and. Copper Hydroxide Electrolyte.
From www.slideshare.net
Electrolysis and Electroplating Copper Hydroxide Electrolyte Effects of electrolyte solution (i.e., koh, naoh, nacl, and hcl) with various concentrations (from 0 to 0.71 m) on corrosion. To which electrode of a direct current power supply should the object be connected? What mass of copper will be deposited if a current of 0.22 amp flows through the cell for 1.5 hours? To date, copper is the only. Copper Hydroxide Electrolyte.
From www.reddit.com
I think this is the final iteration of my copper hydroxide cell. The Copper Hydroxide Electrolyte To date, copper is the only heterogeneous catalyst that has shown a propensity to produce valuable hydrocarbons and alcohols, such as ethylene and ethanol, from electrochemical co 2 reduction (co 2 r). I've been experimenting using electroplating, and i recently made a solution by placing two copper pieces connected to a. A metallic object to be plated with copper is. Copper Hydroxide Electrolyte.
From www.researchgate.net
1 The distribution of arsenite and thioarsenic species in a reducing Copper Hydroxide Electrolyte Copper (ii) hydroxides, particularly cu (oh) 2 micro/nanostructures, are metastable under normal conditions. To date, copper is the only heterogeneous catalyst that has shown a propensity to produce valuable hydrocarbons and alcohols, such as ethylene and ethanol, from electrochemical co 2 reduction (co 2 r). Effects of electrolyte solution (i.e., koh, naoh, nacl, and hcl) with various concentrations (from 0. Copper Hydroxide Electrolyte.
From www.youtube.com
How to Write the Formula for Copper (II) hydroxide YouTube Copper Hydroxide Electrolyte Copper (ii) hydroxides, particularly cu (oh) 2 micro/nanostructures, are metastable under normal conditions. I've been experimenting using electroplating, and i recently made a solution by placing two copper pieces connected to a. A metallic object to be plated with copper is placed in a solution of cuso 4. What mass of copper will be deposited if a current of 0.22. Copper Hydroxide Electrolyte.
From www.researchgate.net
Schematic illustrations of the copper hydroxidebased approach for Copper Hydroxide Electrolyte What mass of copper will be deposited if a current of 0.22 amp flows through the cell for 1.5 hours? I've been experimenting using electroplating, and i recently made a solution by placing two copper pieces connected to a. To which electrode of a direct current power supply should the object be connected? To date, copper is the only heterogeneous. Copper Hydroxide Electrolyte.
From www.scribd.com
Synthesis of Copper Hydroxide3 PDF Stoichiometry Unit Processes Copper Hydroxide Electrolyte To which electrode of a direct current power supply should the object be connected? I've been experimenting using electroplating, and i recently made a solution by placing two copper pieces connected to a. To date, copper is the only heterogeneous catalyst that has shown a propensity to produce valuable hydrocarbons and alcohols, such as ethylene and ethanol, from electrochemical co. Copper Hydroxide Electrolyte.
From www.researchgate.net
Absorption spectra of Cu ions eluted from copper foils in mixed Copper Hydroxide Electrolyte Copper (ii) hydroxides, particularly cu (oh) 2 micro/nanostructures, are metastable under normal conditions. What mass of copper will be deposited if a current of 0.22 amp flows through the cell for 1.5 hours? I've been experimenting using electroplating, and i recently made a solution by placing two copper pieces connected to a. To which electrode of a direct current power. Copper Hydroxide Electrolyte.
From www.alamy.com
Diagram of the electrolysis of copper (II) chromate (VI) showing the Copper Hydroxide Electrolyte To date, copper is the only heterogeneous catalyst that has shown a propensity to produce valuable hydrocarbons and alcohols, such as ethylene and ethanol, from electrochemical co 2 reduction (co 2 r). Copper (ii) hydroxides, particularly cu (oh) 2 micro/nanostructures, are metastable under normal conditions. To which electrode of a direct current power supply should the object be connected? Effects. Copper Hydroxide Electrolyte.
From psu.pb.unizin.org
17.7 Electrolysis Chemistry 112 Chapters 1217 of OpenStax General Copper Hydroxide Electrolyte I've been experimenting using electroplating, and i recently made a solution by placing two copper pieces connected to a. Copper (ii) hydroxides, particularly cu (oh) 2 micro/nanostructures, are metastable under normal conditions. To which electrode of a direct current power supply should the object be connected? Effects of electrolyte solution (i.e., koh, naoh, nacl, and hcl) with various concentrations (from. Copper Hydroxide Electrolyte.
From www.ceramic-glazes.com
Copper Hydroxide Cupric hydroxide patina for ceramics Copper Hydroxide Electrolyte A metallic object to be plated with copper is placed in a solution of cuso 4. I've been experimenting using electroplating, and i recently made a solution by placing two copper pieces connected to a. To which electrode of a direct current power supply should the object be connected? To date, copper is the only heterogeneous catalyst that has shown. Copper Hydroxide Electrolyte.
From blog.thepipingmart.com
Electrolytic Refining of Copper An Overview Copper Hydroxide Electrolyte To date, copper is the only heterogeneous catalyst that has shown a propensity to produce valuable hydrocarbons and alcohols, such as ethylene and ethanol, from electrochemical co 2 reduction (co 2 r). I've been experimenting using electroplating, and i recently made a solution by placing two copper pieces connected to a. To which electrode of a direct current power supply. Copper Hydroxide Electrolyte.
From mungfali.com
Electrolysis Process Diagram Copper Hydroxide Electrolyte A metallic object to be plated with copper is placed in a solution of cuso 4. What mass of copper will be deposited if a current of 0.22 amp flows through the cell for 1.5 hours? I've been experimenting using electroplating, and i recently made a solution by placing two copper pieces connected to a. Effects of electrolyte solution (i.e.,. Copper Hydroxide Electrolyte.
From testbook.com
Copper Hydroxide Learn Definition, Structure, Formula, Uses here Copper Hydroxide Electrolyte To date, copper is the only heterogeneous catalyst that has shown a propensity to produce valuable hydrocarbons and alcohols, such as ethylene and ethanol, from electrochemical co 2 reduction (co 2 r). What mass of copper will be deposited if a current of 0.22 amp flows through the cell for 1.5 hours? Effects of electrolyte solution (i.e., koh, naoh, nacl,. Copper Hydroxide Electrolyte.
From testbook.com
[Solved] In electrolytic refining of copper, the electrolyte is a sol Copper Hydroxide Electrolyte What mass of copper will be deposited if a current of 0.22 amp flows through the cell for 1.5 hours? A metallic object to be plated with copper is placed in a solution of cuso 4. Copper (ii) hydroxides, particularly cu (oh) 2 micro/nanostructures, are metastable under normal conditions. Effects of electrolyte solution (i.e., koh, naoh, nacl, and hcl) with. Copper Hydroxide Electrolyte.
From 2012books.lardbucket.org
Electrochemistry Copper Hydroxide Electrolyte To which electrode of a direct current power supply should the object be connected? What mass of copper will be deposited if a current of 0.22 amp flows through the cell for 1.5 hours? Effects of electrolyte solution (i.e., koh, naoh, nacl, and hcl) with various concentrations (from 0 to 0.71 m) on corrosion. To date, copper is the only. Copper Hydroxide Electrolyte.
From www.youtube.com
Mixing Copper Hydroxide and Barium Sulfate YouTube Copper Hydroxide Electrolyte To which electrode of a direct current power supply should the object be connected? A metallic object to be plated with copper is placed in a solution of cuso 4. To date, copper is the only heterogeneous catalyst that has shown a propensity to produce valuable hydrocarbons and alcohols, such as ethylene and ethanol, from electrochemical co 2 reduction (co. Copper Hydroxide Electrolyte.