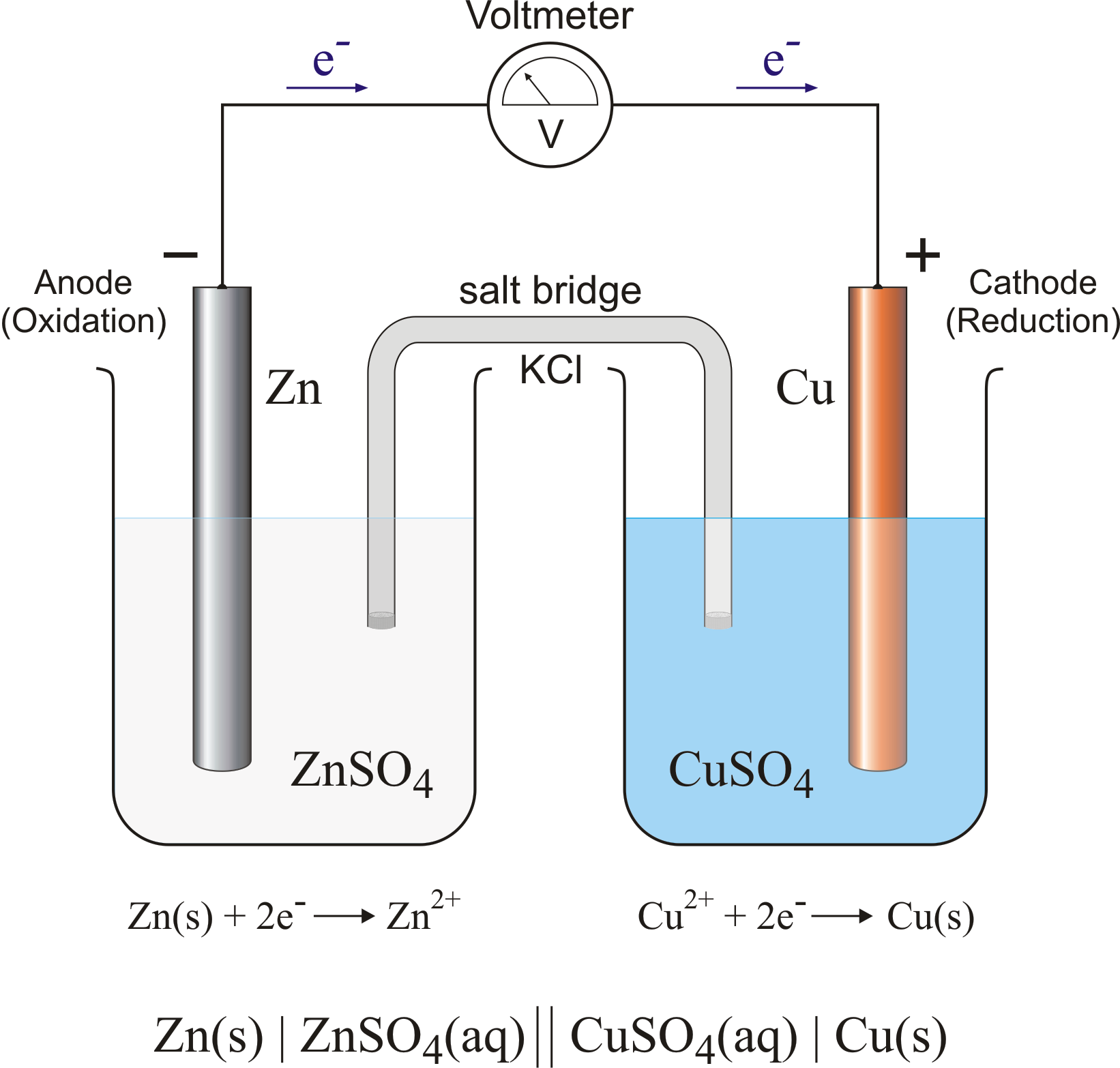
Difference Between Electrolytic Cell And Galvanic Cell Shaalaa . In a galvanic (voltaic) cell a spontaneous chemical reaction produces electricity. Electrolytic and galvanic cells are two types of electrochemical cells, but they operate in opposite ways: In these cells, chemical energy is. Galvanic cells generate electrical energy through spontaneous redox reactions, while. The main difference between the two is their function. Galvanic cells and electrolytic cells are two types of electrochemical cells and have several differences: A galvanic cell is an electrochemical cell that can produce electricity using a chemical reaction. Fundamental difference between an electrolytic cell and the galvanic cell is that in electrolytic cell the free energy is supplied in. A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow.
from glossary.periodni.com
A galvanic cell is an electrochemical cell that can produce electricity using a chemical reaction. In these cells, chemical energy is. In a galvanic (voltaic) cell a spontaneous chemical reaction produces electricity. The main difference between the two is their function. Galvanic cells generate electrical energy through spontaneous redox reactions, while. A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow. Fundamental difference between an electrolytic cell and the galvanic cell is that in electrolytic cell the free energy is supplied in. Electrolytic and galvanic cells are two types of electrochemical cells, but they operate in opposite ways: Galvanic cells and electrolytic cells are two types of electrochemical cells and have several differences:
Galvanic Chemistry Dictionary & Glossary
Difference Between Electrolytic Cell And Galvanic Cell Shaalaa A galvanic cell is an electrochemical cell that can produce electricity using a chemical reaction. Galvanic cells and electrolytic cells are two types of electrochemical cells and have several differences: Fundamental difference between an electrolytic cell and the galvanic cell is that in electrolytic cell the free energy is supplied in. A galvanic cell is an electrochemical cell that can produce electricity using a chemical reaction. In a galvanic (voltaic) cell a spontaneous chemical reaction produces electricity. In these cells, chemical energy is. Electrolytic and galvanic cells are two types of electrochemical cells, but they operate in opposite ways: A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow. The main difference between the two is their function. Galvanic cells generate electrical energy through spontaneous redox reactions, while.
From www.slideserve.com
PPT Galvanic Cells PowerPoint Presentation, free download ID3222668 Difference Between Electrolytic Cell And Galvanic Cell Shaalaa Galvanic cells and electrolytic cells are two types of electrochemical cells and have several differences: A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow. Fundamental difference between an electrolytic cell and the galvanic cell is that in electrolytic cell the free energy is supplied in. In a galvanic (voltaic) cell a spontaneous. Difference Between Electrolytic Cell And Galvanic Cell Shaalaa.
From glossary.periodni.com
Galvanic Chemistry Dictionary & Glossary Difference Between Electrolytic Cell And Galvanic Cell Shaalaa A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow. Electrolytic and galvanic cells are two types of electrochemical cells, but they operate in opposite ways: The main difference between the two is their function. In a galvanic (voltaic) cell a spontaneous chemical reaction produces electricity. Galvanic cells and electrolytic cells are two. Difference Between Electrolytic Cell And Galvanic Cell Shaalaa.
From mavink.com
Differentiate Between Electrochemical Cell And Electrolytic Cell Difference Between Electrolytic Cell And Galvanic Cell Shaalaa A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow. In these cells, chemical energy is. A galvanic cell is an electrochemical cell that can produce electricity using a chemical reaction. Galvanic cells and electrolytic cells are two types of electrochemical cells and have several differences: The main difference between the two is. Difference Between Electrolytic Cell And Galvanic Cell Shaalaa.
From www.geeksforgeeks.org
Electrochemical Cell Definition, Types, Working Principle & Uses Difference Between Electrolytic Cell And Galvanic Cell Shaalaa Electrolytic and galvanic cells are two types of electrochemical cells, but they operate in opposite ways: A galvanic cell is an electrochemical cell that can produce electricity using a chemical reaction. In a galvanic (voltaic) cell a spontaneous chemical reaction produces electricity. The main difference between the two is their function. Fundamental difference between an electrolytic cell and the galvanic. Difference Between Electrolytic Cell And Galvanic Cell Shaalaa.
From www.differencebetween.net
Difference Between Galvanic Cells and Electrolytic Cells Difference Difference Between Electrolytic Cell And Galvanic Cell Shaalaa Galvanic cells generate electrical energy through spontaneous redox reactions, while. A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow. Electrolytic and galvanic cells are two types of electrochemical cells, but they operate in opposite ways: Fundamental difference between an electrolytic cell and the galvanic cell is that in electrolytic cell the free. Difference Between Electrolytic Cell And Galvanic Cell Shaalaa.
From www.studocu.com
Difference Between Electrolytic Cell and Electrochemical Electrolytic Difference Between Electrolytic Cell And Galvanic Cell Shaalaa In a galvanic (voltaic) cell a spontaneous chemical reaction produces electricity. A galvanic cell is an electrochemical cell that can produce electricity using a chemical reaction. Electrolytic and galvanic cells are two types of electrochemical cells, but they operate in opposite ways: Galvanic cells generate electrical energy through spontaneous redox reactions, while. In these cells, chemical energy is. A galvanic. Difference Between Electrolytic Cell And Galvanic Cell Shaalaa.
From mygreexampreparation.com
Galvanic vs Electrolytic Cell MCAT (Electrochemistry Guide) Difference Between Electrolytic Cell And Galvanic Cell Shaalaa A galvanic cell is an electrochemical cell that can produce electricity using a chemical reaction. The main difference between the two is their function. Galvanic cells and electrolytic cells are two types of electrochemical cells and have several differences: A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow. Galvanic cells generate electrical. Difference Between Electrolytic Cell And Galvanic Cell Shaalaa.
From www.differencebetween.com
Difference Between Electrochemical Cell and Galvanic Cell Compare the Difference Between Electrolytic Cell And Galvanic Cell Shaalaa A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow. In these cells, chemical energy is. The main difference between the two is their function. A galvanic cell is an electrochemical cell that can produce electricity using a chemical reaction. In a galvanic (voltaic) cell a spontaneous chemical reaction produces electricity. Galvanic cells. Difference Between Electrolytic Cell And Galvanic Cell Shaalaa.
From testbook.com
Know Difference Between Galvanic Cells And Electrolytic Cells Difference Between Electrolytic Cell And Galvanic Cell Shaalaa In these cells, chemical energy is. Fundamental difference between an electrolytic cell and the galvanic cell is that in electrolytic cell the free energy is supplied in. A galvanic cell is an electrochemical cell that can produce electricity using a chemical reaction. Galvanic cells generate electrical energy through spontaneous redox reactions, while. The main difference between the two is their. Difference Between Electrolytic Cell And Galvanic Cell Shaalaa.
From ar.inspiredpencil.com
Galvanic Cell Vs Electrolytic Cell Difference Between Electrolytic Cell And Galvanic Cell Shaalaa Fundamental difference between an electrolytic cell and the galvanic cell is that in electrolytic cell the free energy is supplied in. In a galvanic (voltaic) cell a spontaneous chemical reaction produces electricity. A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow. A galvanic cell is an electrochemical cell that can produce electricity. Difference Between Electrolytic Cell And Galvanic Cell Shaalaa.
From www.vrogue.co
Voltaic Vs Galvanic Cell vrogue.co Difference Between Electrolytic Cell And Galvanic Cell Shaalaa Fundamental difference between an electrolytic cell and the galvanic cell is that in electrolytic cell the free energy is supplied in. A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow. A galvanic cell is an electrochemical cell that can produce electricity using a chemical reaction. Electrolytic and galvanic cells are two types. Difference Between Electrolytic Cell And Galvanic Cell Shaalaa.
From jesse-has-lozano.blogspot.com
Explain the Difference Between a Galvanic Cell and Electrolytic Cell Difference Between Electrolytic Cell And Galvanic Cell Shaalaa A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow. In a galvanic (voltaic) cell a spontaneous chemical reaction produces electricity. In these cells, chemical energy is. The main difference between the two is their function. Fundamental difference between an electrolytic cell and the galvanic cell is that in electrolytic cell the free. Difference Between Electrolytic Cell And Galvanic Cell Shaalaa.
From jackwestin.com
Galvanic Or Voltaic Cells Electrochemistry MCAT Content Difference Between Electrolytic Cell And Galvanic Cell Shaalaa Electrolytic and galvanic cells are two types of electrochemical cells, but they operate in opposite ways: The main difference between the two is their function. In these cells, chemical energy is. Fundamental difference between an electrolytic cell and the galvanic cell is that in electrolytic cell the free energy is supplied in. Galvanic cells generate electrical energy through spontaneous redox. Difference Between Electrolytic Cell And Galvanic Cell Shaalaa.
From ar.inspiredpencil.com
Galvanic Cell Vs Electrolytic Cell Difference Between Electrolytic Cell And Galvanic Cell Shaalaa Electrolytic and galvanic cells are two types of electrochemical cells, but they operate in opposite ways: In a galvanic (voltaic) cell a spontaneous chemical reaction produces electricity. Galvanic cells generate electrical energy through spontaneous redox reactions, while. A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow. A galvanic cell is an electrochemical. Difference Between Electrolytic Cell And Galvanic Cell Shaalaa.
From differguide.com
Difference Between Galvanic And Electrolytic Cell Difference Between Electrolytic Cell And Galvanic Cell Shaalaa In a galvanic (voltaic) cell a spontaneous chemical reaction produces electricity. A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow. The main difference between the two is their function. Galvanic cells and electrolytic cells are two types of electrochemical cells and have several differences: Electrolytic and galvanic cells are two types of. Difference Between Electrolytic Cell And Galvanic Cell Shaalaa.
From www.slideserve.com
PPT Galvanic and Electrolytic Cells PowerPoint Presentation, free Difference Between Electrolytic Cell And Galvanic Cell Shaalaa Fundamental difference between an electrolytic cell and the galvanic cell is that in electrolytic cell the free energy is supplied in. Galvanic cells and electrolytic cells are two types of electrochemical cells and have several differences: In a galvanic (voltaic) cell a spontaneous chemical reaction produces electricity. In these cells, chemical energy is. A galvanic cell is an electrochemical cell. Difference Between Electrolytic Cell And Galvanic Cell Shaalaa.
From ar.inspiredpencil.com
Galvanic Cell Vs Electrolytic Cell Difference Between Electrolytic Cell And Galvanic Cell Shaalaa In a galvanic (voltaic) cell a spontaneous chemical reaction produces electricity. Galvanic cells and electrolytic cells are two types of electrochemical cells and have several differences: Electrolytic and galvanic cells are two types of electrochemical cells, but they operate in opposite ways: The main difference between the two is their function. In these cells, chemical energy is. A galvanic cell. Difference Between Electrolytic Cell And Galvanic Cell Shaalaa.
From www.youtube.com
What is Electrochemical Cell Notation Line notation Cell Diagram Difference Between Electrolytic Cell And Galvanic Cell Shaalaa Fundamental difference between an electrolytic cell and the galvanic cell is that in electrolytic cell the free energy is supplied in. In these cells, chemical energy is. Galvanic cells and electrolytic cells are two types of electrochemical cells and have several differences: A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow. Galvanic. Difference Between Electrolytic Cell And Galvanic Cell Shaalaa.
From thechemistrynotes.com
Difference and Similarity Galvanic vs Electrolytic Cell Difference Between Electrolytic Cell And Galvanic Cell Shaalaa Fundamental difference between an electrolytic cell and the galvanic cell is that in electrolytic cell the free energy is supplied in. Electrolytic and galvanic cells are two types of electrochemical cells, but they operate in opposite ways: A galvanic cell is an electrochemical cell that can produce electricity using a chemical reaction. In a galvanic (voltaic) cell a spontaneous chemical. Difference Between Electrolytic Cell And Galvanic Cell Shaalaa.
From www.youtube.com
Galvanic Cell Vs Electrolytic Cell differences YouTube Difference Between Electrolytic Cell And Galvanic Cell Shaalaa In a galvanic (voltaic) cell a spontaneous chemical reaction produces electricity. Galvanic cells generate electrical energy through spontaneous redox reactions, while. Electrolytic and galvanic cells are two types of electrochemical cells, but they operate in opposite ways: Galvanic cells and electrolytic cells are two types of electrochemical cells and have several differences: A galvanic cell is an electrochemical cell in. Difference Between Electrolytic Cell And Galvanic Cell Shaalaa.
From www.vrogue.co
What Is The Difference Between Galvanic Cell And Elec vrogue.co Difference Between Electrolytic Cell And Galvanic Cell Shaalaa The main difference between the two is their function. Galvanic cells generate electrical energy through spontaneous redox reactions, while. In a galvanic (voltaic) cell a spontaneous chemical reaction produces electricity. In these cells, chemical energy is. A galvanic cell is an electrochemical cell that can produce electricity using a chemical reaction. A galvanic cell is an electrochemical cell in which. Difference Between Electrolytic Cell And Galvanic Cell Shaalaa.
From pediaa.com
Difference Between Electrochemical Cell and Electrolytic Cell Difference Between Electrolytic Cell And Galvanic Cell Shaalaa Galvanic cells and electrolytic cells are two types of electrochemical cells and have several differences: A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow. The main difference between the two is their function. Galvanic cells generate electrical energy through spontaneous redox reactions, while. In a galvanic (voltaic) cell a spontaneous chemical reaction. Difference Between Electrolytic Cell And Galvanic Cell Shaalaa.
From in.pinterest.com
three different types of electronic devices are shown in this diagram Difference Between Electrolytic Cell And Galvanic Cell Shaalaa Galvanic cells and electrolytic cells are two types of electrochemical cells and have several differences: A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow. Galvanic cells generate electrical energy through spontaneous redox reactions, while. Fundamental difference between an electrolytic cell and the galvanic cell is that in electrolytic cell the free energy. Difference Between Electrolytic Cell And Galvanic Cell Shaalaa.
From www.youtube.com
Difference between Electrolytic and Galvanic cells YouTube Difference Between Electrolytic Cell And Galvanic Cell Shaalaa Galvanic cells and electrolytic cells are two types of electrochemical cells and have several differences: In a galvanic (voltaic) cell a spontaneous chemical reaction produces electricity. A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow. Fundamental difference between an electrolytic cell and the galvanic cell is that in electrolytic cell the free. Difference Between Electrolytic Cell And Galvanic Cell Shaalaa.
From pediaa.com
Difference Between Galvanic and Electrolytic Cell Definition, How Difference Between Electrolytic Cell And Galvanic Cell Shaalaa In a galvanic (voltaic) cell a spontaneous chemical reaction produces electricity. Electrolytic and galvanic cells are two types of electrochemical cells, but they operate in opposite ways: Galvanic cells and electrolytic cells are two types of electrochemical cells and have several differences: The main difference between the two is their function. Fundamental difference between an electrolytic cell and the galvanic. Difference Between Electrolytic Cell And Galvanic Cell Shaalaa.
From chemwiki.ucdavis.edu
Electrolytic Cells Chemwiki Difference Between Electrolytic Cell And Galvanic Cell Shaalaa Fundamental difference between an electrolytic cell and the galvanic cell is that in electrolytic cell the free energy is supplied in. The main difference between the two is their function. A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow. In these cells, chemical energy is. Galvanic cells generate electrical energy through spontaneous. Difference Between Electrolytic Cell And Galvanic Cell Shaalaa.
From www.javatpoint.com
Difference Between Galvanic Cells and Electrolytic Cells javatpoint Difference Between Electrolytic Cell And Galvanic Cell Shaalaa Galvanic cells generate electrical energy through spontaneous redox reactions, while. The main difference between the two is their function. A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow. A galvanic cell is an electrochemical cell that can produce electricity using a chemical reaction. Galvanic cells and electrolytic cells are two types of. Difference Between Electrolytic Cell And Galvanic Cell Shaalaa.
From askanydifference.com
Galvanic Cells vs Electrolytic Cells Difference and Comparison Difference Between Electrolytic Cell And Galvanic Cell Shaalaa Galvanic cells and electrolytic cells are two types of electrochemical cells and have several differences: The main difference between the two is their function. In a galvanic (voltaic) cell a spontaneous chemical reaction produces electricity. Galvanic cells generate electrical energy through spontaneous redox reactions, while. Electrolytic and galvanic cells are two types of electrochemical cells, but they operate in opposite. Difference Between Electrolytic Cell And Galvanic Cell Shaalaa.
From www.youtube.com
Comparison of Electrolytic and Galvanic Cells Chy9 Chp7 Difference Between Electrolytic Cell And Galvanic Cell Shaalaa Electrolytic and galvanic cells are two types of electrochemical cells, but they operate in opposite ways: Galvanic cells generate electrical energy through spontaneous redox reactions, while. The main difference between the two is their function. A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow. In these cells, chemical energy is. Fundamental difference. Difference Between Electrolytic Cell And Galvanic Cell Shaalaa.
From ar.inspiredpencil.com
Galvanic Cell Vs Electrolytic Cell Difference Between Electrolytic Cell And Galvanic Cell Shaalaa Galvanic cells and electrolytic cells are two types of electrochemical cells and have several differences: Electrolytic and galvanic cells are two types of electrochemical cells, but they operate in opposite ways: Fundamental difference between an electrolytic cell and the galvanic cell is that in electrolytic cell the free energy is supplied in. Galvanic cells generate electrical energy through spontaneous redox. Difference Between Electrolytic Cell And Galvanic Cell Shaalaa.
From www.yaclass.in
Types of Electrochemical Cell and Electrolytic Cell — lesson. Science Difference Between Electrolytic Cell And Galvanic Cell Shaalaa The main difference between the two is their function. A galvanic cell is an electrochemical cell that can produce electricity using a chemical reaction. Electrolytic and galvanic cells are two types of electrochemical cells, but they operate in opposite ways: In these cells, chemical energy is. Galvanic cells generate electrical energy through spontaneous redox reactions, while. Galvanic cells and electrolytic. Difference Between Electrolytic Cell And Galvanic Cell Shaalaa.
From www.youtube.com
Comparison between the electrolytic cell and chemical cell YouTube Difference Between Electrolytic Cell And Galvanic Cell Shaalaa Fundamental difference between an electrolytic cell and the galvanic cell is that in electrolytic cell the free energy is supplied in. A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow. A galvanic cell is an electrochemical cell that can produce electricity using a chemical reaction. Galvanic cells generate electrical energy through spontaneous. Difference Between Electrolytic Cell And Galvanic Cell Shaalaa.
From www.youtube.com
Galvanic Cell vs Electrolytic Cell animation Electrochemical Cells Difference Between Electrolytic Cell And Galvanic Cell Shaalaa Electrolytic and galvanic cells are two types of electrochemical cells, but they operate in opposite ways: In these cells, chemical energy is. Galvanic cells generate electrical energy through spontaneous redox reactions, while. In a galvanic (voltaic) cell a spontaneous chemical reaction produces electricity. Galvanic cells and electrolytic cells are two types of electrochemical cells and have several differences: A galvanic. Difference Between Electrolytic Cell And Galvanic Cell Shaalaa.
From keyesvannuyshyundai.blogspot.com
draw the diagram of electrolytic cell and explain keyesvannuyshyundai Difference Between Electrolytic Cell And Galvanic Cell Shaalaa In these cells, chemical energy is. A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow. Fundamental difference between an electrolytic cell and the galvanic cell is that in electrolytic cell the free energy is supplied in. Galvanic cells generate electrical energy through spontaneous redox reactions, while. In a galvanic (voltaic) cell a. Difference Between Electrolytic Cell And Galvanic Cell Shaalaa.
From www.youtube.com
What is the Difference between Galvanic cell and Electrolytic cell Difference Between Electrolytic Cell And Galvanic Cell Shaalaa In these cells, chemical energy is. A galvanic cell is an electrochemical cell that can produce electricity using a chemical reaction. The main difference between the two is their function. In a galvanic (voltaic) cell a spontaneous chemical reaction produces electricity. Galvanic cells generate electrical energy through spontaneous redox reactions, while. A galvanic cell is an electrochemical cell in which. Difference Between Electrolytic Cell And Galvanic Cell Shaalaa.